Wave Life Sciences unveils Phase I results for obesity treatment
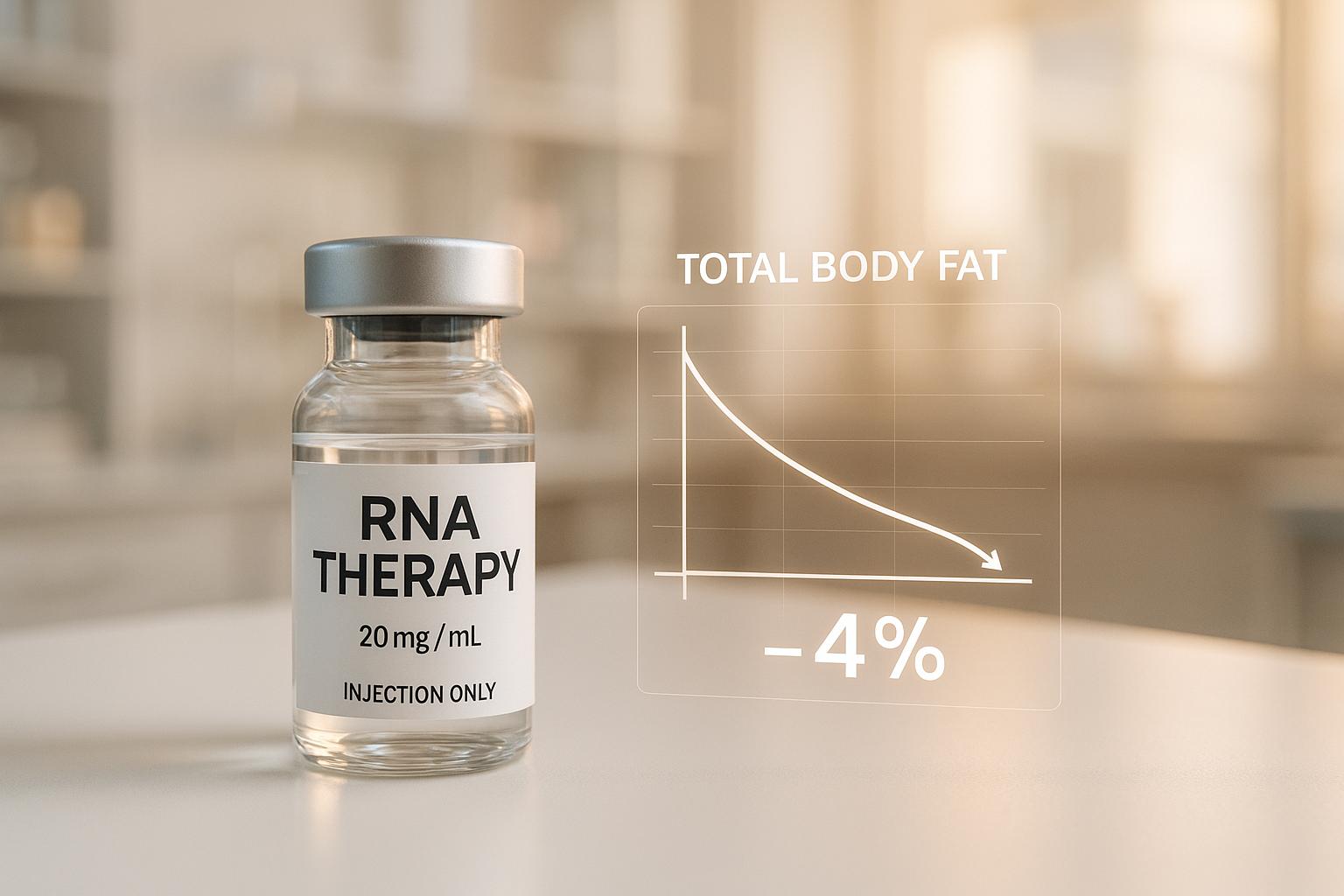
Wave Life Sciences has announced interim data from its Phase I INLIGHT trial, revealing promising early results for its injectable RNA treatment, WVE-007. A single dose of the drug reportedly reduced total body fat by 4% after approximately three months, sparking optimism about its potential to reshape the obesity treatment landscape.
The trial’s findings include a placebo-adjusted 9.2% reduction in visceral fat and a 0.9% increase in lean mass at the lowest therapeutic dose. Unlike other weight loss treatments, such as GLP-1-based therapies, WVE-007 demonstrated no statistically significant loss of lean muscle, a challenge that has led to the development of muscle-preserving add-on therapies in this space.
"Bottomline, in our view, update today from WVE-007 fundamentally changes the outlook for obesity landscape in a very disruptive manner and for the better", analysts at Truist commented. They described the results as "impressive" and noted the preservation of lean mass as a notable advantage.
Safety Profile and Comparisons
The safety profile of WVE-007 also drew praise, with no serious adverse events or discontinuations reported in the Phase I trial. It was particularly noted for avoiding the gastrointestinal side effects commonly associated with GLP-1 treatments. "Safety looks very clean", wrote analysts at Mizuho Group, who further emphasized the drug’s potential advantages over Novo Nordisk’s semaglutide, marketed as Wegovy for weight loss and Ozempic for diabetes. At the 12-week mark in prior trials, semaglutide produced 2–2.5% fat loss but also resulted in a 3.5% reduction in lean mass, according to the analysts.
Mizuho Group projections estimate peak sales of WVE-007 could reach around $7 billion if the drug ultimately secures approval.
Market Reaction and Upcoming Data
Following the announcement, shares of Wave Life Sciences surged by 80%, reaching $13.52 in early trading. Anticipation is now building for additional data from the trial. In the first and second quarters of 2026, Wave plans to release six-month data for the 240 mg dose, three- and six-month data for a 400 mg dose, and three-month data for a 600 mg dose.
Mechanism of Action
WVE-007 operates as an injectable RNAi therapy, targeting the mRNA encoding the INHBE protein. Wave Life Sciences explained that individuals with a naturally occurring loss-of-function mutation in one copy of the INHBE gene often exhibit improved body composition and a healthier cardiometabolic profile.
As the trial progresses, analysts remain optimistic about the drug’s potential. "The results bode well for longer-term data", said analysts at Truist, who look forward to further updates from the ongoing study early next year.
Read the source

Transforming Lives, One Step at a Time
Keep reading
How to Adjust Weight Loss Goals on GLP-1 Medications
Adjust weight goals on GLP-1 meds by tracking body composition and labs, optimizing protein, activity, sleep, and working with your provider on dosing.
Falsified Mounjaro pens prompt urgent safety advisory
MHRA warns of counterfeit Mounjaro pens (batch D873576); stop use and check batch numbers for infection risk.
Novo Nordisk shares drop as Alzheimer’s hopes for weight-loss drug fade
Novo Nordisk’s semaglutide failed to slow Alzheimer’s in large trials, prompting shares to fall and expert reactions.