GLP-1 vs GIP: Understanding Dual Agonist Medications

If you’ve researched weight loss medications, you’ve probably seen references to GLP-1 agonists like semaglutide and dual GLP-1/GIP agonists like tirzepatide. Maybe you understand that tirzepatide acts on two hormone receptors while semaglutide acts on one, but the practical significance might not be entirely clear.
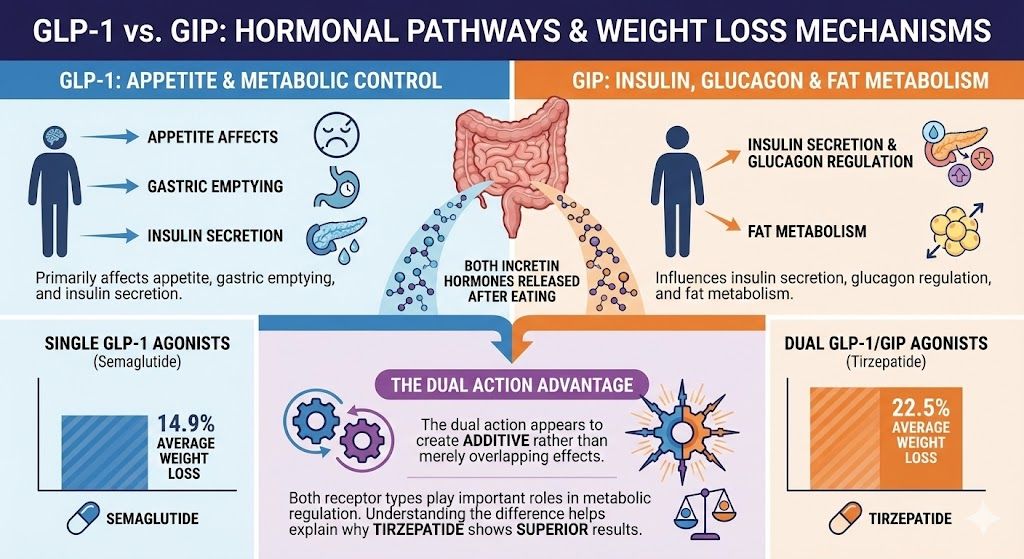
Here’s what you need to know: GLP-1 and GIP are both incretin hormones your gut releases after eating. They work through related but distinct mechanisms to regulate appetite, blood sugar, and metabolism. Medications that activate just GLP-1 receptors produce excellent weight loss results. Medications that activate both GLP-1 and GIP receptors produce even better results, with tirzepatide showing 22.5% average weight loss compared to semaglutide’s 14.9%.
This guide explains what GLP-1 and GIP actually are, how they differ in function, why combining both creates additive benefits, and what this means for choosing between medications.
Key Takeaways: GLP-1 vs GIP
- Both are incretin hormones released by your intestines after eating
- GLP-1 primarily affects appetite, gastric emptying, and insulin secretion
- GIP influences insulin secretion, glucagon regulation, and fat metabolism
- Single GLP-1 agonists (semaglutide) produce 14.9% average weight loss
- Dual GLP-1/GIP agonists (tirzepatide) produce 22.5% average weight loss
- The dual action appears to create additive rather than merely overlapping effects
- Both receptor types play important roles in metabolic regulation
- Understanding the difference helps explain why tirzepatide shows superior results
What Are Incretin Hormones?
Before diving into GLP-1 versus GIP specifically, understanding incretin hormones as a category provides helpful context.
Incretins are hormones your intestines secrete when you eat food. They travel through your bloodstream to various organs, triggering responses that help your body handle the incoming nutrients. The two main incretins are GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide, previously called gastric inhibitory polypeptide).
These hormones serve multiple functions in metabolic regulation. They stimulate insulin release from your pancreas when blood sugar rises, helping move glucose from your blood into your cells. They suppress glucagon secretion, preventing your liver from producing more glucose when you’ve just eaten. They slow how quickly food moves from your stomach into your intestines. They affect appetite and satiety signals in your brain.
Scientists discovered that people with type 2 diabetes have blunted incretin responses compared to people without diabetes. This observation sparked interest in developing medications that could boost incretin activity, potentially treating both diabetes and obesity.
The challenge was that natural incretins break down within minutes of release. Enzymes in your blood, particularly DPP-4, rapidly degrade them. Early medications that simply boosted natural incretin levels had limited effectiveness because the hormones still degraded quickly.
The breakthrough came from creating synthetic versions of these hormones engineered to resist breakdown. These medications bind to the same receptors as natural incretins but last for hours or days instead of minutes. This extended duration made practical treatment possible.
Understanding GLP-1: The First Target
GLP-1 became the first incretin targeted for pharmaceutical development, leading to medications like exenatide, liraglutide, and eventually semaglutide.
What GLP-1 Does in Your Body
Your intestinal L-cells secrete GLP-1 in response to food, particularly when you eat carbohydrates, fats, and proteins. Once released, GLP-1 travels through your bloodstream and activates GLP-1 receptors located throughout your body.
The most important effects for weight loss happen in your brain. GLP-1 receptors in your hypothalamus and other brain regions control appetite and satiety. When activated, these receptors reduce hunger signals and increase feelings of fullness. This is why GLP-1 medications so effectively suppress appetite. You naturally want to eat less because your brain is receiving stronger “I’m satisfied” signals.
GLP-1 also significantly slows gastric emptying. Your stomach holds food longer before releasing it into your small intestine. This prolonged stomach retention contributes to feeling full longer after eating. It’s why many people on GLP-1 medications report that meals satisfy them for hours when they used to feel hungry again within an hour or two.
In your pancreas, GLP-1 stimulates insulin secretion in a glucose-dependent manner. This means it only triggers insulin release when blood sugar is elevated, reducing the risk of hypoglycemia. It also suppresses glucagon, the hormone that tells your liver to produce more glucose. This combination helps lower blood sugar levels.
Some evidence suggests GLP-1 might also affect reward pathways in the brain related to food cravings and the pleasure response to eating. Many people report that foods they previously craved intensely lose their appeal on GLP-1 medications. That constant mental preoccupation with food, what some call “food noise,” often quiets substantially.
GLP-1 Receptor Distribution
GLP-1 receptors exist throughout your body, not just in one or two locations. You have them in your brain (hypothalamus, brainstem), pancreas (beta cells and alpha cells), stomach, intestines, heart, and kidneys. This widespread distribution means GLP-1 affects multiple systems simultaneously.
The brain receptors drive the appetite suppression that produces weight loss. The pancreatic receptors improve blood sugar control. The stomach receptors slow gastric emptying. The cardiovascular receptors may provide heart protection benefits observed in some clinical trials.
This multi-system impact explains why GLP-1 medications produce benefits beyond just weight loss. Many people see improvements in blood pressure, cholesterol levels, inflammation markers, and cardiovascular risk factors.
GLP-1 Medications
Several medications work by activating GLP-1 receptors. Semaglutide (Ozempic for diabetes, Wegovy for weight loss) represents the most effective pure GLP-1 agonist currently available. Other GLP-1 medications include liraglutide (Saxenda, Victoza), dulaglutide (Trulicity), and exenatide (Byetta, Bydureon).
These medications all work through the same basic mechanism of activating GLP-1 receptors. They differ in potency, duration of action, and specific receptor binding characteristics, but they’re fundamentally doing the same thing: mimicking and amplifying your body’s natural GLP-1 signals.
For comprehensive information about semaglutide specifically, see our complete guide to semaglutide weight loss results.
Understanding GIP: The Second Incretin
GIP received less attention initially than GLP-1 because early research suggested it might not be useful for diabetes treatment. However, newer understanding revealed GIP’s important metabolic roles.
What GIP Does in Your Body
Your intestinal K-cells secrete GIP in response to eating, particularly when you consume fats and carbohydrates. Like GLP-1, GIP travels through your bloodstream and activates specific GIP receptors in various organs.
In your pancreas, GIP strongly stimulates insulin secretion when blood glucose is elevated. In fact, GIP may be an even more potent insulin secretagogue than GLP-1 under certain conditions. It also appears to support beta cell health and survival, potentially helping preserve pancreatic function over time.
Interestingly, GIP affects glucagon differently than GLP-1. While GLP-1 suppresses glucagon, GIP’s effect on glucagon depends on glucose levels. When blood sugar is low, GIP can actually increase glucagon secretion, which helps prevent hypoglycemia. When blood sugar is high, GIP has minimal effect on glucagon. This glucose-dependent modulation provides a built-in safety mechanism.
GIP also plays important roles in fat metabolism. GIP receptors exist on fat cells, and activating them appears to influence how your body stores and uses fat. Early research suggested GIP might promote fat storage, which initially made scientists skeptical about using GIP agonists for weight loss. However, newer evidence suggests the relationship is more complex and that GIP activation in the context of GLP-1 activation might actually support fat burning.
Unlike GLP-1, GIP doesn’t significantly slow gastric emptying. Food moves through your stomach at normal speed when only GIP receptors are activated. This is one key difference between the two incretins.
GIP’s effects on appetite and brain signaling are less clear than GLP-1’s. GIP receptors exist in some brain regions, but their role in appetite regulation isn’t as well-established. Some research suggests GIP might influence food reward pathways, but this remains an active area of investigation.
GIP Receptor Distribution
GIP receptors are found in your pancreas (beta cells and alpha cells), fat tissue (adipocytes), bone, brain (limited distribution compared to GLP-1), stomach, and intestines.
The pancreatic receptors drive the insulin secretion effects. The fat tissue receptors influence lipid metabolism and storage. The bone receptors may affect bone formation and turnover. The brain receptors, though less numerous than GLP-1 receptors, might contribute to metabolic regulation through central mechanisms.
This distribution suggests GIP influences metabolism through somewhat different pathways than GLP-1, even though both are incretin hormones.
Why GIP Was Initially Controversial
Early studies in people with type 2 diabetes found that their insulin response to GIP was diminished compared to people without diabetes. This led researchers to wonder if GIP agonists would even work in the population that needed them most.
Additionally, some animal studies suggested GIP promoted fat accumulation, raising concerns that GIP agonists might cause weight gain rather than loss.
These concerns kept GIP on the back burner for years while GLP-1 took center stage in drug development. Only when Eli Lilly began testing dual GLP-1/GIP agonists did the picture change dramatically.
The Dual Agonist Breakthrough: Why Two Is Better Than One
Tirzepatide, the first dual GLP-1/GIP agonist to reach market, demonstrated that activating both receptors produces superior results to activating GLP-1 alone.
Clinical Trial Evidence
The head-to-head comparison comes from the SURPASS clinical trial program for diabetes and SURMOUNT program for weight loss. In SURMOUNT-1, tirzepatide at 15 mg weekly produced 22.5% average weight loss over 72 weeks. This vastly exceeded the 14.9% average weight loss with semaglutide 2.4 mg weekly in the STEP-1 trial.
While these weren’t direct head-to-head trials (different studies, different populations), the magnitude of difference is striking. We’re talking about roughly 50% more weight loss with tirzepatide compared to semaglutide.
In direct comparison trials for diabetes (SURPASS-2 compared tirzepatide to semaglutide 1 mg), tirzepatide showed superior blood sugar control and greater weight loss. Patients on tirzepatide lost more weight at every dose level compared to semaglutide.
This consistent pattern across multiple trials suggests the dual agonist approach offers genuine advantages over single GLP-1 agonism.
Theories About Why Dual Action Works Better
Scientists are still working to fully understand why adding GIP agonism to GLP-1 agonism creates such substantial additional benefits. Several theories exist:
The additive effects theory suggests that GIP and GLP-1 work through partially independent pathways. GLP-1 provides powerful appetite suppression through brain receptors. GIP adds metabolic improvements through pancreatic and fat tissue effects. Together, they attack weight and metabolic dysfunction from multiple angles simultaneously.
The synergistic enhancement theory proposes that GIP and GLP-1 don’t just add to each other but actually amplify each other’s effects. GIP might make tissues more responsive to GLP-1, or vice versa. The combined activation might trigger metabolic changes that neither hormone produces alone.
The central nervous system theory focuses on brain effects. While GLP-1 clearly affects appetite centers in the brain, adding GIP agonism might influence different neural circuits related to food reward, energy expenditure, or metabolic regulation. The two hormones together might create a more comprehensive effect on brain-regulated metabolism.
The fat metabolism theory suggests GIP’s effects on adipose tissue are crucial. In the context of simultaneous GLP-1 activation and caloric restriction, GIP might help mobilize fat stores more effectively or prevent metabolic adaptations that normally fight weight loss.
The glucose-dependent effects theory highlights that both hormones work in glucose-dependent ways but with different patterns. Their combined action might produce more precise, effective metabolic regulation than either achieves alone.
Likely, multiple mechanisms contribute. The dual agonist approach isn’t about one magic bullet but rather about comprehensive activation of complementary metabolic pathways.
For detailed comparison of outcomes with these medications, see our Mounjaro vs Ozempic comparison.
Practical Differences: What This Means for Treatment
Understanding GLP-1 versus GIP matters when choosing between medications or understanding why one might work better for you than another.
Side Effect Profiles
The most noticeable practical difference is that dual agonists like tirzepatide tend to cause slightly more gastrointestinal side effects than pure GLP-1 agonists. Nausea, diarrhea, and digestive discomfort occur somewhat more frequently with tirzepatide than with semaglutide.
This likely relates to the combined effects of both hormones on the digestive system. While GIP doesn’t slow gastric emptying like GLP-1 does, it still affects gut function. The dual activation creates a stronger overall impact on your GI tract.
However, the difference isn’t dramatic. Many people tolerate tirzepatide just fine with manageable side effects. The slightly higher side effect rate is a tradeoff for significantly better weight loss results.
Appetite Suppression Differences
Most people report powerful appetite suppression on both pure GLP-1 agonists and dual agonists. However, some individuals notice that tirzepatide creates even stronger appetite reduction than semaglutide.
This might relate to GIP’s potential effects on food reward pathways or metabolic signaling. When both receptor systems are activated, the cumulative impact on appetite and food thoughts might exceed what GLP-1 alone produces.
Anecdotally, some people who switched from semaglutide to tirzepatide report that their appetite, which had started returning somewhat on semaglutide, decreased again on tirzepatide. This suggests the dual mechanism might provide additional appetite control beyond GLP-1 alone.
Metabolic Effects Beyond Weight Loss
Both medication types improve metabolic markers, but dual agonists might offer some advantages in certain areas.
Blood sugar control appears slightly better with tirzepatide in head-to-head trials, possibly due to GIP’s robust insulin secretion effects. Lipid profiles (cholesterol and triglycerides) might improve more with dual agonists. Some evidence suggests dual agonists might better preserve lean body mass during weight loss, though this remains under investigation.
These metabolic differences are generally modest compared to the dramatic difference in total weight loss. However, for people with significant metabolic dysfunction like type 2 diabetes, the enhanced metabolic benefits might matter.
Cost Considerations
Brand-name dual agonists (Mounjaro/Zepbound) cost $1,069 monthly compared to $969 to $1,349 for pure GLP-1 agonists (Ozempic/Wegovy). Both are expensive without insurance.
In compounded form, the cost difference is more manageable. Compounded tirzepatide costs $349 per month through TrimRx, while compounded semaglutide costs $199 per month. The $150 monthly difference ($1,800 annually) is significant but not as prohibitive as the brand-name cost difference.
For many people, the decision comes down to whether the additional weight loss potential justifies the extra cost. Some start with semaglutide given its lower price and excellent results, then switch to tirzepatide if they plateau or want to maximize outcomes.
The Science Behind Dual Agonist Design
Creating tirzepatide required sophisticated pharmaceutical engineering to activate both receptors effectively.
The Challenge of Dual Activation
Simply combining a GLP-1 agonist with a GIP agonist wouldn’t necessarily work well. You’d need to take two separate medications, each with its own dosing schedule and side effect profile. The goal was creating a single molecule that could activate both receptors.
The challenge is that GLP-1 and GIP are structurally similar but not identical. A molecule that fits perfectly into GLP-1 receptors might not activate GIP receptors effectively, and vice versa. Eli Lilly needed to design a molecule with the right balance of activity at both receptors.
How Tirzepatide Achieves Balance
Tirzepatide is based on the GIP molecular structure but modified to also activate GLP-1 receptors effectively. It binds to both receptor types, though with different affinities.
Tirzepatide actually has higher affinity for GIP receptors than GLP-1 receptors at the molecular level. However, the dosing and pharmacokinetics are designed so that both receptors receive clinically meaningful activation.
The molecule also includes modifications that extend its half-life to about 5 days, enabling once-weekly dosing. These same types of modifications (fatty acid chains, albumin binding) that work for pure GLP-1 agonists also extend tirzepatide’s duration.
The end result is a single molecule that can be injected once weekly and produces robust activation of both incretin pathways.
Future Directions
Tirzepatide represents just the beginning of multi-agonist approaches. Pharmaceutical companies are developing triple agonists that add glucagon agonism to GLP-1 and GIP. Early trials suggest these might produce even greater weight loss, potentially approaching 30% or more.
Other companies are exploring different combinations of hormone agonists, different dosing strategies, and oral formulations of dual agonists.
The concept of attacking metabolic dysfunction through multiple complementary pathways simultaneously appears to be the future direction of obesity pharmacotherapy.
GLP-1 vs GIP: Which Matters More?
An interesting question is whether one of these hormones contributes more to tirzepatide’s effects than the other.
The GLP-1 Dominance Hypothesis
Some researchers believe GLP-1 agonism drives most of the weight loss benefit, with GIP adding a modest boost. This view suggests that semaglutide captures most of the available benefit from incretin-based therapy, and tirzepatide’s advantage comes from fine-tuning rather than fundamentally different mechanisms.
Supporting this view is the fact that pure GLP-1 agonists work very well. Semaglutide’s 14.9% average weight loss is substantial. Pure GIP agonists, when tested without GLP-1 agonism, haven’t shown impressive weight loss results in early studies.
This suggests GLP-1 is the key player for appetite suppression and weight loss, with GIP providing enhancement rather than being equally important.
The Synergy Hypothesis
Others believe the magic is specifically in the combination. Neither hormone alone produces what you see with dual agonism. GLP-1 provides the appetite suppression foundation, but GIP fundamentally changes how the body responds metabolically in ways that dramatically amplify weight loss beyond what GLP-1 alone achieves.
Supporting this view is the magnitude of tirzepatide’s advantage. A 50% increase in weight loss compared to semaglutide (22.5% vs 14.9%) seems too large to explain as mere enhancement. It suggests GIP is doing something substantial beyond just adding a small boost.
The Likely Reality
Both hormones probably matter, but in different ways. GLP-1 provides the strong appetite suppression that makes eating less feasible and sustainable. GIP provides metabolic optimization that maximizes fat loss and minimizes metabolic adaptation.
Together, they create a more comprehensive effect than either achieves alone. GLP-1 might be more important for total appetite control, but GIP’s contribution to the overall weight loss magnitude is clearly significant.
The fact remains that dual agonism beats single agonism in clinical trials. Whether GIP contributes 30% of the benefit or 50% matters less than the reality that adding it produces substantially better results.
Choosing Between GLP-1 and Dual Agonist Medications
Armed with understanding of GLP-1 versus GIP, how do you decide which medication type to use?
Start with GLP-1 If
You want excellent results at lower cost. Semaglutide produces substantial weight loss at $199 monthly in compounded form. You’re concerned about side effects and prefer starting with the medication that tends to have slightly milder GI issues. Your insurance covers GLP-1 medications but not dual agonists. You want to see how you respond to incretin-based therapy before committing to the more expensive option.
Many people achieve their weight loss goals with semaglutide and see no reason to escalate to tirzepatide. The 14.9% average weight loss is enough for their needs.
Choose Dual Agonist If
You want to maximize weight loss potential from the start. You tried GLP-1 medications and plateaued before reaching your goal. You have significant metabolic dysfunction and want the broadest possible metabolic benefits. Cost difference is manageable for your budget. You’re comfortable with potentially slightly higher side effect rates for better results.
For detailed comparison of these medication options, see our Zepbound vs Wegovy guide.
The Sequential Approach
A reasonable strategy is starting with semaglutide and escalating to tirzepatide if needed. This lets you capture the benefits of GLP-1 agonism at lower cost. If you achieve your goals on semaglutide, perfect. If weight loss stalls before reaching your target, switching to tirzepatide often restarts progress.
This approach also helps you understand how your body responds to incretin-based therapy. You’ll know whether you tolerate these medications well, how much appetite suppression you experience, and what kind of weight loss pace to expect.
Some people alternate between the two medications over time, using semaglutide for maintenance and tirzepatide for periods when they need additional weight loss.
The Future of Incretin-Based Therapy
Understanding GLP-1 versus GIP provides context for where this field is heading.
Triple Agonists
Adding glucagon agonism to GLP-1/GIP agonism creates triple agonists currently in clinical trials. Early results suggest these might produce 25% to 30% average weight loss, potentially exceeding even tirzepatide.
Glucagon affects energy expenditure and fat metabolism through different pathways than GLP-1 or GIP. Adding it to the mix might create yet another additive or synergistic benefit.
However, glucagon agonism also comes with its own side effects and considerations. The risk-benefit profile of triple agonists remains under investigation.
Selective Agonism
Rather than activating receptors fully, some experimental medications partially activate them or activate specific subtypes. This might allow fine-tuning the benefit-to-side-effect ratio.
For example, a medication might strongly activate brain GLP-1 receptors for appetite suppression while only partially activating gut receptors that contribute to nausea.
Oral Formulations
Currently, all approved incretin medications require injection because the peptide molecules break down in your digestive system. However, oral semaglutide exists for diabetes treatment, using special absorption enhancers.
Oral versions of dual agonists are in development. If successful, they could dramatically improve convenience and potentially increase treatment uptake.
Combination Approaches
Future treatments might combine incretin agonists with other weight loss mechanisms like metabolism boosters, appetite suppressants working through non-incretin pathways, or fat absorption inhibitors.
Multi-pronged approaches attacking obesity through completely independent mechanisms simultaneously might produce even better results than current single-medication approaches.
Frequently Asked Questions
What is the difference between GLP-1 and GIP?
Both are incretin hormones your gut releases after eating, but they differ in function. GLP-1 strongly suppresses appetite, slows gastric emptying, and stimulates insulin while suppressing glucagon. GIP primarily stimulates insulin secretion, affects glucagon in glucose-dependent ways, and influences fat metabolism. GLP-1 has stronger effects on appetite and brain signaling, while GIP has more pronounced effects on fat tissue and glucose-dependent metabolic regulation.
Why does tirzepatide work better than semaglutide?
Tirzepatide activates both GLP-1 and GIP receptors, while semaglutide only activates GLP-1 receptors. The dual action creates additive or potentially synergistic effects that produce greater weight loss. Clinical trials show tirzepatide produces 22.5% average weight loss compared to semaglutide’s 14.9%, likely because activating both incretin pathways provides more comprehensive metabolic regulation than activating one pathway alone.
Which is more important for weight loss, GLP-1 or GIP?
GLP-1 appears more important for appetite suppression and probably drives the majority of weight loss effect. Pure GLP-1 agonists work very well on their own. However, adding GIP agonism significantly enhances results, suggesting GIP contributes meaningfully to overall weight loss through metabolic effects beyond pure appetite control. Both hormones matter, but GLP-1 is likely the primary driver with GIP providing substantial enhancement.
Are dual agonists better than single GLP-1 agonists?
Dual agonists produce more weight loss on average in clinical trials, making them objectively more effective for total weight loss. However, “better” depends on individual priorities. Dual agonists cost more and may cause slightly more side effects. Single GLP-1 agonists produce excellent results at lower cost with potentially milder side effects. For maximizing weight loss, dual agonists are better. For balancing effectiveness with cost and tolerability, single agonists might be better for some people.
Do GLP-1 and GIP medications have different side effects?
Both cause similar side effects, primarily gastrointestinal issues like nausea, diarrhea, and constipation. Dual agonists that activate both GLP-1 and GIP receptors tend to cause these side effects slightly more frequently than pure GLP-1 agonists. The difference isn’t dramatic, but rates of nausea and diarrhea are modestly higher with tirzepatide compared to semaglutide in clinical trials. Most people tolerate both medication types well after initial adjustment.
Can you take separate GLP-1 and GIP medications together?
Currently, no pure GIP agonist medications are approved for clinical use. Tirzepatide is engineered as a single molecule that activates both receptors rather than being two separate medications combined. Taking a GLP-1 medication plus a hypothetical GIP medication separately wouldn’t necessarily produce the same effects as a designed dual agonist, and this approach hasn’t been studied.
Does GIP slow gastric emptying like GLP-1?
No, GIP does not significantly slow gastric emptying. This is one key difference between the two incretins. GLP-1 powerfully slows how quickly your stomach empties food into your intestines, contributing to prolonged fullness. GIP lacks this effect. Dual agonists like tirzepatide slow gastric emptying because of their GLP-1 activity, not their GIP activity.
Will there be more dual agonist medications in the future?
Yes, pharmaceutical companies are developing additional dual and triple agonist medications. These include different GLP-1/GIP combinations with varying receptor affinity profiles, triple agonists that add glucagon activity, and oral formulations of dual agonists. The success of tirzepatide has validated the multi-agonist approach, driving substantial research and development in this area.
Is GIP agonism safe for weight loss?
Clinical trials with tirzepatide demonstrate that GIP agonism combined with GLP-1 agonism is safe and effective for weight loss. Early concerns that GIP might promote fat storage haven’t materialized as problems in actual treatment. The safety profile of dual agonists is similar to pure GLP-1 agonists, with the main difference being slightly higher rates of GI side effects. Long-term safety continues to be monitored as these medications remain relatively new.
Which medication should I choose, semaglutide or tirzepatide?
This depends on your priorities and circumstances. Choose semaglutide if you want excellent results at lower cost ($199 vs $349 monthly for compounded versions), prefer potentially milder side effects, or want to start conservatively. Choose tirzepatide if you want maximum weight loss potential, tried semaglutide and plateaued, or can manage the higher cost. Many people start with semaglutide and switch to tirzepatide if needed, which is a reasonable strategy.
Making Sense of Incretin Science
Understanding the difference between GLP-1 and GIP helps demystify why certain weight loss medications work better than others. These two incretin hormones affect metabolism through related but distinct pathways. GLP-1 provides powerful appetite suppression and gastric slowing. GIP enhances insulin secretion and affects fat metabolism. Together, they create a more comprehensive attack on obesity than either achieves alone.
The dual agonist approach represented by tirzepatide demonstrates that activating multiple complementary pathways produces superior results to targeting a single pathway, even when that single pathway works quite well on its own. This principle is likely to guide future medication development as researchers explore triple agonists and other multi-targeted approaches.
For you as someone considering or using these medications, the practical takeaway is straightforward. Pure GLP-1 agonists like semaglutide work excellently and represent a good starting point for most people. Dual GLP-1/GIP agonists like tirzepatide work even better and make sense when you want to maximize results or haven’t achieved your goals with GLP-1 alone.
Whether you choose compounded semaglutide at $199 monthly or compounded tirzepatide at $349 monthly through TrimRx, you’re accessing medications based on solid incretin science that produces meaningful weight loss results. Get started with an online consultation and begin treatment with medications designed around these powerful metabolic hormones.

Transforming Lives, One Step at a Time
Keep reading
GLP-1 for Type 2 Diabetes: Beyond Blood Sugar Control
When GLP-1 medications first emerged for Type 2 diabetes, they were one option among many for lowering blood sugar. Today, they’ve become the cornerstone…
Ozempic Face: Causes, Prevention, and What You Need to Know
If you’ve spent any time researching Ozempic or semaglutide, you’ve probably encountered the term “Ozempic face.” Maybe you’ve seen dramatic before-and-after photos online showing…
Does Semaglutide Make You Tired? Fatigue Causes and Solutions
If you’re taking semaglutide or considering starting it, you might be wondering whether the medication will leave you feeling exhausted. Maybe you’ve heard people…



