Ozempic and Insulin Resistance: How It Works and Expected Results

Insulin resistance sits at the center of many metabolic problems. It drives Type 2 diabetes, contributes to weight gain, worsens PCOS, and increases cardiovascular risk. If you have insulin resistance, you’ve probably been told to lose weight and exercise more. Maybe you’ve tried metformin. And now you’re wondering whether Ozempic, the medication producing dramatic weight loss results, might help with the underlying insulin resistance itself.
The answer is yes, and in multiple ways. Semaglutide (the active ingredient in Ozempic) improves insulin resistance through both direct metabolic effects and indirect effects mediated by weight loss. For many patients, this dual mechanism produces more significant improvement in insulin sensitivity than other available treatments.
Here’s what makes semaglutide particularly valuable for insulin resistance: Unlike medications that only address blood sugar or only promote weight loss, semaglutide does both while also directly improving how your body responds to insulin. This means improvements in metabolic function that go beyond what weight loss alone would produce, with effects that show up in measurable lab values, reduced medication needs, and often in how you feel day to day.
This guide covers:
- What insulin resistance actually is and why it matters
- The specific mechanisms through which semaglutide improves insulin sensitivity
- What clinical research shows about metabolic improvements
- Timeline for seeing results in lab values and symptoms
- How improvements relate to weight loss
- Comparing semaglutide to metformin and other treatments
- Who benefits most from semaglutide for insulin resistance
- Monitoring your progress and understanding your lab results
- Long-term implications for metabolic health
Key Takeaways
- Semaglutide improves insulin resistance through multiple mechanisms, including direct effects on glucose metabolism and indirect effects through weight loss
- Improvements begin within weeks, with measurable changes in fasting glucose often appearing before significant weight loss
- Clinical trials show significant reductions in HbA1c (1.0-1.6 percentage points on average), fasting glucose, and fasting insulin levels
- Weight loss amplifies metabolic benefits, with each 5% of body weight lost producing meaningful additional improvement in insulin sensitivity
- Effects extend beyond blood sugar, improving blood pressure, cholesterol, liver fat, and inflammatory markers
- Many patients reduce or eliminate diabetes medications, including some who discontinue insulin entirely
- Semaglutide generally produces greater metabolic improvement than metformin, particularly for weight loss, though both medications have roles
- Prediabetes often reverses, with many patients returning to normal glucose tolerance
- Benefits require ongoing treatment, as insulin resistance typically worsens again if medication is stopped and weight regains
- Compounded semaglutide produces equivalent metabolic effects at lower cost ($199/month through TrimRx)
Understanding Insulin Resistance
Before exploring how semaglutide helps, understanding what insulin resistance actually is provides essential context.
What Happens in Insulin Resistance
Insulin is a hormone produced by the pancreas that allows cells to take up glucose (sugar) from the bloodstream for energy. In a healthy metabolic system, cells respond readily to insulin’s signal, blood sugar stays stable, and the pancreas produces modest amounts of insulin to maintain balance.
In insulin resistance, cells become less responsive to insulin’s signal. It’s like the cells have become hard of hearing and need insulin to “speak louder” before they respond. The pancreas compensates by producing more insulin, sometimes much more, to force the same glucose uptake.
This compensation works for a while. Blood sugar may stay normal even as insulin levels climb higher and higher. But the elevated insulin causes problems of its own, and eventually the pancreas can’t keep up. When insulin production can no longer compensate for resistance, blood sugar rises and prediabetes or Type 2 diabetes develops.
Why Insulin Resistance Matters
The consequences of insulin resistance extend far beyond blood sugar:
Metabolic effects: High insulin promotes fat storage, particularly visceral fat around organs. It makes weight loss more difficult and weight gain more likely. It increases triglycerides and decreases HDL cholesterol.
Cardiovascular effects: Insulin resistance is a major driver of cardiovascular disease, independent of whether it progresses to diabetes. Elevated insulin damages blood vessels, promotes inflammation, and accelerates atherosclerosis.
Liver effects: Insulin resistance contributes to non-alcoholic fatty liver disease (NAFLD), which affects an estimated 25% of adults and can progress to serious liver damage.
Hormonal effects: In women, insulin resistance drives elevated androgens, contributing to PCOS and its symptoms. In men, it can affect testosterone levels and reproductive function.
Inflammatory effects: Insulin resistance is associated with chronic low-grade inflammation, which contributes to various disease processes.
Cancer risk: Some research links insulin resistance and hyperinsulinemia to increased risk of certain cancers.
Addressing insulin resistance isn’t just about preventing diabetes. It’s about reducing risk across multiple organ systems and improving overall metabolic health.
How Insulin Resistance Develops
Multiple factors contribute to insulin resistance:
Excess weight: Particularly visceral fat (around organs in the midsection), which is metabolically active and releases substances that impair insulin signaling.
Sedentary lifestyle: Physical inactivity reduces insulin sensitivity. Muscle tissue is a major site of glucose disposal, and inactive muscles become less responsive.
Genetics: Some people are genetically predisposed to insulin resistance, independent of weight or lifestyle.
Diet: Diets high in refined carbohydrates, added sugars, and processed foods may contribute. Chronic overnutrition stresses metabolic systems.
Age: Insulin sensitivity tends to decrease with age, though lifestyle factors matter more than age itself.
Sleep deprivation: Chronic insufficient sleep impairs glucose metabolism and insulin sensitivity.
Stress: Chronic stress elevates cortisol, which promotes insulin resistance.
Inflammation: Chronic inflammation impairs insulin signaling at the cellular level.
Most people with significant insulin resistance have multiple contributing factors. Treatment approaches that address several factors simultaneously tend to be most effective.
How Semaglutide Improves Insulin Resistance
Semaglutide affects insulin resistance through several distinct mechanisms, both direct and indirect.
Direct Metabolic Effects
Semaglutide is a GLP-1 (glucagon-like peptide-1) receptor agonist. GLP-1 is a hormone naturally produced in the gut after eating, and it has multiple effects on glucose metabolism:
Enhanced insulin secretion: GLP-1 stimulates the pancreas to release insulin, but only when blood sugar is elevated. This glucose-dependent action reduces hypoglycemia risk compared to medications that stimulate insulin release regardless of blood sugar levels.
Suppressed glucagon: GLP-1 reduces the release of glucagon, a hormone that signals the liver to release stored glucose. By suppressing glucagon, semaglutide reduces the liver’s contribution to elevated blood sugar.
Reduced hepatic glucose production: Beyond glucagon suppression, GLP-1 agonists decrease the liver’s production and release of glucose through additional mechanisms.
Beta cell protection: Some evidence suggests GLP-1 agonists may help protect and preserve pancreatic beta cells (the cells that produce insulin), potentially slowing the progression of diabetes.
Improved insulin sensitivity: Research indicates GLP-1 agonists improve how cells respond to insulin, independent of weight loss. The mechanisms aren’t fully understood but may involve effects on cellular signaling pathways and reduced inflammation.
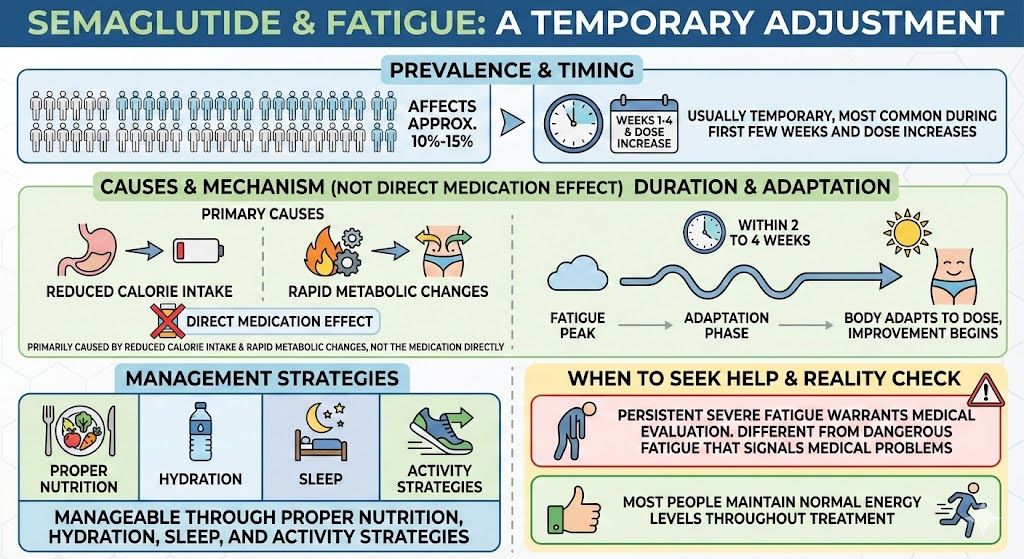
These direct effects mean semaglutide improves glucose metabolism even before significant weight loss occurs. Patients often see improvements in fasting glucose and post-meal glucose within the first few weeks of treatment.
Weight Loss Effects
Semaglutide produces substantial weight loss, averaging 15% of body weight in clinical trials. This weight loss powerfully improves insulin resistance:
Reduced visceral fat: Semaglutide reduces visceral fat more than some other interventions, and visceral fat is particularly associated with insulin resistance. Losing visceral fat removes a major driver of metabolic dysfunction.
Decreased inflammation: Weight loss reduces the chronic inflammation associated with obesity, which improves insulin signaling at the cellular level.
Improved muscle insulin sensitivity: As excess fat decreases, muscle tissue becomes more responsive to insulin.
Reduced ectopic fat: Fat stored in organs like the liver (fatty liver disease) decreases with weight loss, improving those organs’ metabolic function.
Lower insulin demand: With less body mass to fuel and improved insulin sensitivity, the pancreas doesn’t need to produce as much insulin. This reduces the strain on beta cells and lowers circulating insulin levels.
The weight loss effects amplify and compound the direct metabolic effects. Patients who achieve significant weight loss typically see greater improvement in insulin resistance than those with minimal weight loss, even with the same medication.
Appetite and Behavior Effects
Semaglutide’s effects on appetite and eating behavior indirectly support metabolic improvement:
Reduced caloric intake: Lower appetite leads to eating less, creating the caloric deficit that produces weight loss and its metabolic benefits.
Improved food choices: Many patients report reduced cravings for high-sugar, high-fat foods. This dietary shift supports better glucose control beyond simple calorie reduction.
Slowed gastric emptying: By slowing how quickly food leaves the stomach, semaglutide reduces post-meal glucose spikes. The same meal produces a lower, more gradual rise in blood sugar.
Reduced “food noise”: The mental quieting of food preoccupation allows patients to make more deliberate food choices rather than responding to constant hunger signals.
What Clinical Research Shows
Clinical trials provide concrete data on what metabolic improvements to expect.
Blood Sugar Improvements
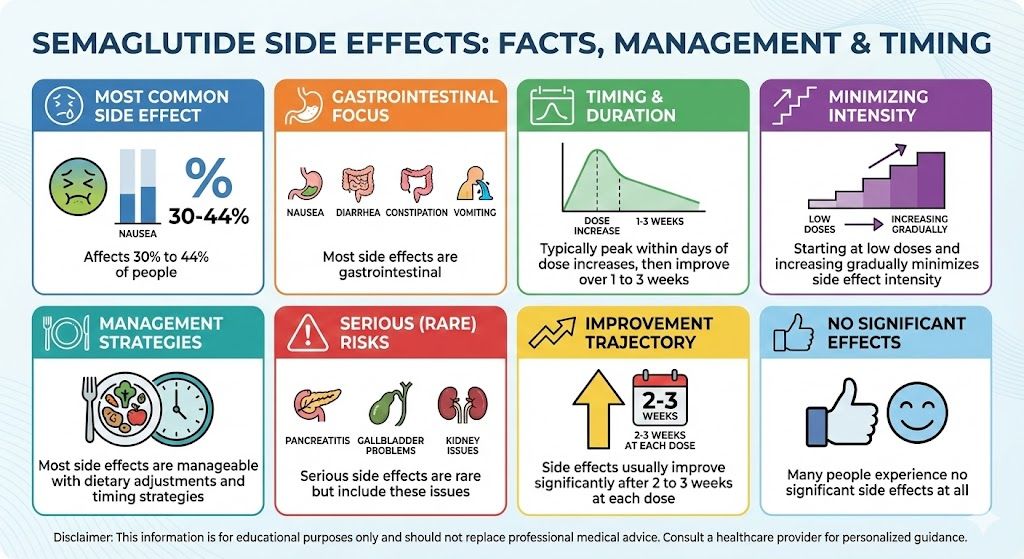
Across multiple clinical trials, semaglutide consistently produces significant improvements in glucose control:
HbA1c reductions: In patients with Type 2 diabetes, semaglutide reduces HbA1c by approximately 1.0-1.6 percentage points on average. For someone starting at 8.0%, this could mean reaching 6.5-7.0%, potentially meeting treatment targets.
Fasting glucose: Fasting glucose typically decreases by 20-40 mg/dL on average, though individual responses vary.
Post-meal glucose: The glucose spikes after eating are substantially reduced, improving overall glucose control beyond what fasting numbers alone show.
Time in range: For patients using continuous glucose monitors, time spent in the target glucose range increases significantly.
Insulin Level Changes
Changes in insulin levels reflect improved insulin sensitivity:
Fasting insulin: Typically decreases as cells become more responsive and less insulin is needed to maintain glucose control.
HOMA-IR: This calculated measure of insulin resistance (using fasting glucose and insulin) improves significantly in most patients.
Post-meal insulin: The insulin response to meals often becomes more efficient, with lower peaks and better return to baseline.
Results in Prediabetes
For patients with prediabetes (elevated blood sugar that hasn’t reached diabetic levels), semaglutide often produces normalization:
Return to normal glucose: Many prediabetic patients achieve normal fasting glucose and HbA1c levels.
Diabetes prevention: By improving insulin resistance and glucose control, semaglutide may prevent or significantly delay progression to Type 2 diabetes.
No dedicated prediabetes trials: While semaglutide isn’t specifically approved for prediabetes, the metabolic improvements it produces clearly benefit this population.
Results in Established Diabetes
For patients with Type 2 diabetes, benefits are well-documented:
Medication reduction: Many patients reduce the number or doses of diabetes medications. Some patients on multiple medications are able to simplify to semaglutide alone.
Insulin reduction or discontinuation: Patients taking insulin often reduce doses substantially. Some patients who required insulin achieve good enough control with semaglutide to discontinue insulin entirely.
Improved control despite reduced medication: The combination of improved insulin sensitivity plus semaglutide’s direct effects often produces better glucose control than previous multi-drug regimens.
Comparison to Metformin
Metformin has been the first-line treatment for insulin resistance and Type 2 diabetes for decades. How does semaglutide compare?
| Factor | Semaglutide | Metformin |
| HbA1c reduction | 1.0-1.6% | 0.5-1.0% |
| Weight loss | 10-15% | 2-5% |
| Fasting glucose reduction | 20-40 mg/dL | 15-25 mg/dL |
| Cardiovascular benefit | Proven reduction in events | Suggested but less proven |
| Cost (without insurance) | $199-349/month | $4-20/month |
| Administration | Weekly injection | Daily pills |
| GI side effects | Common initially | Common initially |
Semaglutide generally produces greater metabolic improvement, but metformin remains valuable:
- Much lower cost makes it accessible regardless of insurance
- Decades of safety data
- Can be combined with semaglutide for enhanced effect
- Appropriate first-line option when cost is prohibitive
Many providers use both medications, starting metformin for initial treatment and adding semaglutide when additional improvement is needed or when weight loss is a priority.
Timeline for Metabolic Improvement
Understanding when to expect improvements helps set realistic expectations.
First Two Weeks
Even before significant weight loss, early metabolic changes occur:
Blood sugar stabilization: Many patients notice more stable blood sugar, with fewer highs and lows, within the first week or two.
Reduced post-meal spikes: The slowing of gastric emptying begins immediately, reducing glucose excursions after meals.
Appetite changes: The reduction in hunger that eventually produces weight loss begins during this period.
Lab changes: Too early for meaningful HbA1c change, but fasting glucose may already begin decreasing.
First Month
Weight loss begins: Typically 2-5 pounds, contributing modestly to metabolic improvement.
Fasting glucose: Often measurably improved, sometimes by 10-20 mg/dL or more.
Medication adjustments: Patients on insulin or sulfonylureas may need dose reductions to prevent hypoglycemia.
Subjective changes: Some patients report feeling better, with more stable energy levels.
Months Two to Three
Accelerating weight loss: Moving through dose increases, weight loss accelerates. Cumulative loss often 8-15 pounds.
More significant glucose improvements: Fasting glucose continues decreasing. Post-meal control improves further.
Early HbA1c changes: HbA1c begins reflecting cumulative improvement, though it takes 2-3 months for the full effect of glucose changes to show in HbA1c.
Medication reductions: More significant reductions in other diabetes medications may be appropriate.
Months Three to Six
Substantial metabolic improvement: This is when the combination of weight loss and direct medication effects produces dramatic results.
HbA1c: Typically shows maximum improvement, reflecting sustained glucose control over the preceding months. Reductions of 1.0-1.5 percentage points are common.
Weight loss: Often 15-25 pounds cumulative, amplifying metabolic benefits.
Insulin sensitivity: Measurably improved on testing. Patients who were highly insulin resistant often show substantial improvement.
Other markers: Triglycerides, blood pressure, liver enzymes all typically improving.
Months Six to Twelve
Continued improvement: Metabolic markers continue improving as weight loss continues, though rate of improvement slows.
Stabilization: Some patients reach new stable levels as weight loss plateaus.
Maximum benefit: By month 12, most patients have achieved the bulk of their metabolic improvement.
Long-term management: Focus shifts from active improvement to maintaining gains.
Beyond Year One
Maintenance phase: With continued treatment, metabolic improvements are typically maintained.
Ongoing benefit: Patients continue benefiting from improved insulin sensitivity as long as treatment continues.
Progressive diabetes: For some diabetic patients, the condition may progress despite treatment, eventually requiring additional interventions.
Measuring Your Progress
Tracking the right markers helps you understand how your insulin resistance is responding to treatment.
Key Lab Tests
HbA1c: This test reflects average blood sugar over the past 2-3 months. It’s the standard measure for diabetes management and also useful for tracking prediabetes and insulin resistance.
- Normal: Below 5.7%
- Prediabetes: 5.7-6.4%
- Diabetes: 6.5% or higher
- Goal on treatment varies but typically under 7% for most diabetics, lower for prediabetics
Fasting glucose: Measured after 8 or more hours without eating. Reflects baseline glucose production and insulin sensitivity.
- Normal: Under 100 mg/dL
- Prediabetes: 100-125 mg/dL
- Diabetes: 126 mg/dL or higher
Fasting insulin: Not always ordered but can be useful for assessing insulin resistance. Higher levels suggest more resistance (the pancreas is working harder to maintain glucose control).
- Optimal: Under 10 μU/mL
- Elevated: Over 15-20 μU/mL suggests significant resistance
HOMA-IR: Calculated from fasting glucose and insulin. A more direct measure of insulin resistance.
- Normal: Under 1.0
- Elevated: Over 2.0-2.5 suggests significant resistance
Lipid panel: Triglycerides in particular reflect insulin resistance and typically improve with treatment.
- Optimal triglycerides: Under 150 mg/dL
- High: Over 200 mg/dL
Liver enzymes (ALT, AST): Elevated in fatty liver disease, which is associated with insulin resistance. Often improve with treatment.
Testing Schedule
Before starting treatment: Baseline labs establish your starting point. Include HbA1c, fasting glucose, lipid panel, and liver enzymes at minimum.
Month 3: First meaningful checkpoint. HbA1c reflects the first few months of treatment. Repeat fasting glucose and lipids.
Month 6: Second checkpoint. Should show continued or maximum improvement.
Every 3-6 months ongoing: Regular monitoring to ensure benefits are maintained and adjust other medications as needed.
Additional testing: Your provider may order fasting insulin, HOMA-IR, or other tests based on your specific situation.
Non-Lab Indicators
Beyond lab tests, other indicators suggest improving insulin resistance:
Weight loss: The most obvious indicator. Every 5% of body weight lost typically produces meaningful metabolic improvement.
Waist circumference: Reduction suggests loss of visceral fat, the metabolically problematic fat around organs.
Blood pressure: Often decreases as insulin resistance improves.
Energy levels: Many patients report more stable energy without the highs and crashes associated with blood sugar fluctuations.
Reduced medication needs: If you’re able to reduce doses of diabetes medications while maintaining control, your insulin sensitivity is improving.
Skin changes: Acanthosis nigricans (dark, velvety skin patches often found on the neck and armpits) may fade as insulin levels decrease.
Who Benefits Most
While semaglutide improves insulin resistance across populations, certain groups may see particularly significant benefits.
Prediabetes
Patients with prediabetes often see dramatic results because they’re intervening before the metabolic dysfunction becomes severe:
Prevention potential: Treating insulin resistance in the prediabetic stage may prevent progression to Type 2 diabetes entirely.
Higher response rate: The pancreas still functions well, so improving insulin sensitivity allows the system to normalize.
Return to normal: Many prediabetic patients achieve normal glucose levels and HbA1c.
Long-term implications: Preventing diabetes prevents all its complications, making early intervention particularly valuable.
Early Type 2 Diabetes
Patients diagnosed with Type 2 diabetes in the past few years often respond well:
Better beta cell function: Earlier in diabetes, pancreatic beta cells are still relatively preserved. Improving insulin sensitivity allows remaining function to be sufficient.
Medication simplification: Many patients can achieve good control with semaglutide alone or with reduced other medications.
Potential “remission”: Some patients achieve glucose levels below diabetic thresholds, though this typically requires ongoing treatment.
PCOS
Women with polycystic ovary syndrome frequently have significant insulin resistance, and semaglutide addresses this core issue:
Hormonal improvement: Reduced insulin levels decrease ovarian androgen production.
Weight loss: Particularly beneficial given PCOS-related weight management difficulties.
Menstrual regularity: Often improves as insulin resistance resolves.
Fertility potential: May improve for some women, though semaglutide must be stopped before pregnancy.
For detailed information on PCOS specifically, see our guide on Ozempic for PCOS.
Obesity With Metabolic Dysfunction
Patients with obesity and insulin resistance, even without formal diabetes diagnosis:
Comprehensive metabolic improvement: Weight loss plus direct effects address multiple aspects of metabolic syndrome.
Risk reduction: Treating insulin resistance reduces cardiovascular risk, cancer risk, and other obesity-associated complications.
Quality of life: Beyond disease prevention, patients often feel significantly better.
Who May See Less Dramatic Results
Some patients may see more modest improvement in insulin resistance:
Advanced Type 2 diabetes: Patients with long-standing diabetes and significant beta cell dysfunction may have less capacity to benefit from improved insulin sensitivity.
On significant insulin doses: While semaglutide often allows insulin reduction, patients requiring high doses may have less capacity for dramatic improvement.
Minimal weight loss: The metabolic benefits of semaglutide are amplified by weight loss. Patients who don’t lose significant weight may see less improvement.
Genetic factors: Some insulin resistance has strong genetic components that may be less responsive to any intervention.
Effects Beyond Insulin Resistance
Semaglutide’s metabolic benefits extend beyond glucose and insulin.
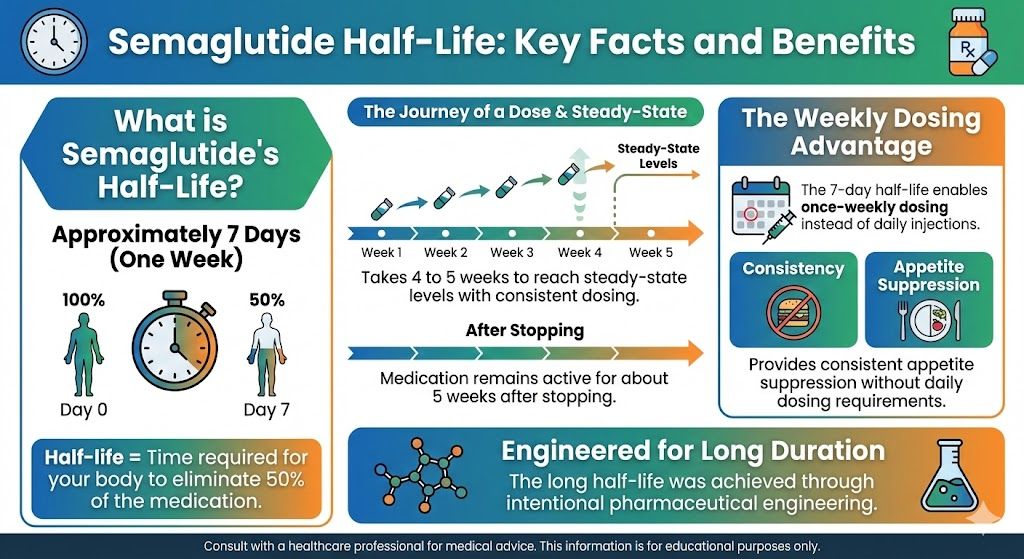
Cardiovascular Effects
Insulin resistance is a major cardiovascular risk factor. Semaglutide improves multiple cardiovascular markers:
Blood pressure: Typically decreases 4-6 mmHg systolic, reducing strain on the heart and blood vessels.
Lipids: Triglycerides often decrease significantly. LDL cholesterol may decrease modestly. These changes reduce atherosclerosis risk.
Inflammation: Markers of inflammation (like C-reactive protein) typically decrease, reducing vascular inflammation.
Cardiovascular events: The SELECT trial demonstrated a 20% reduction in major cardiovascular events (heart attack, stroke, cardiovascular death) with semaglutide in patients at high cardiovascular risk.
Liver Effects
Non-alcoholic fatty liver disease (NAFLD) is closely linked to insulin resistance:
Reduced liver fat: Studies show significant reductions in liver fat content with semaglutide treatment.
Improved liver enzymes: ALT and AST levels typically decrease, suggesting reduced liver inflammation.
Potential disease modification: While more research is needed, semaglutide may slow or reverse NAFLD progression.
Clinical trials ongoing: Studies specifically examining semaglutide for NASH (non-alcoholic steatohepatitis, a more severe form of fatty liver disease) show promising results.
Kidney Effects
Insulin resistance affects kidney function, and semaglutide may provide protection:
Reduced albumin in urine: An early marker of kidney damage often improves with semaglutide.
Preserved kidney function: Some evidence suggests GLP-1 agonists may slow decline in kidney function.
Benefit in diabetic kidney disease: For patients with diabetes-related kidney disease, semaglutide may provide additional protection.
Sleep Apnea
Obstructive sleep apnea is associated with insulin resistance, and both often improve together:
Weight loss effect: Much of the sleep apnea improvement comes from weight loss reducing airway obstruction.
Metabolic effect: Improved insulin resistance may provide additional benefit, as the conditions share underlying mechanisms.
Quality of life: Better sleep improves energy, mood, and overall function.
Practical Considerations
Several practical factors affect how you approach semaglutide treatment for insulin resistance.
Starting Treatment
Eligibility: Standard eligibility requires BMI of 30 or higher, or BMI 27 or higher with a weight-related condition. Insulin resistance, prediabetes, and Type 2 diabetes all count as qualifying conditions.
Provider options: You can pursue semaglutide through primary care physicians, endocrinologists, or telehealth platforms like TrimRx that specialize in weight management.
Initial evaluation: Baseline labs help establish your starting point and identify any contraindications.
For information on accessing treatment, see our guide on how to get prescribed Ozempic.
Cost Considerations
Cost affects treatment access and sustainability:
Brand-name options:
- Ozempic (for diabetes): $349/month through NovoCare cash-pay
- Wegovy (for weight management): $349/month through NovoCare cash-pay
Compounded semaglutide: $199/month through TrimRx, containing the same active ingredient at lower cost.
Insurance: Coverage varies widely. Diabetes diagnosis improves coverage likelihood for Ozempic. Weight loss indications (Wegovy) are less commonly covered.
Long-term planning: Since ongoing treatment is typically necessary, sustainable cost matters. Compounded options make long-term treatment more accessible.
For detailed pricing information, see our guides on compounded semaglutide costs and Ozempic insurance coverage.
Coordinating With Other Diabetes Medications
If you’re already on diabetes medications, starting semaglutide requires coordination:
Sulfonylureas (glipizide, glyburide, glimepiride): Hypoglycemia risk increases when combined with semaglutide. Doses often need reduction when starting.
Insulin: Hypoglycemia risk increases. Insulin doses typically need reduction, sometimes substantially.
Metformin: Generally safe to combine. Some providers continue metformin alongside semaglutide; others discontinue if not needed.
SGLT2 inhibitors (empagliflozin, dapagliflozin): Can be combined, though the combination isn’t always necessary.
DPP-4 inhibitors (sitagliptin, linagliptin): Generally discontinued when starting semaglutide since both affect the incretin system.
Never adjust medications without provider guidance. The coordination requires medical oversight to prevent hypoglycemia and optimize outcomes.
Monitoring for Hypoglycemia
As insulin resistance improves, hypoglycemia (low blood sugar) can become a concern, particularly for patients on insulin or sulfonylureas:
Symptoms: Shakiness, sweating, confusion, rapid heartbeat, dizziness, hunger.
Prevention: Your provider should proactively reduce other diabetes medications as you start semaglutide.
Response: Treat with fast-acting carbohydrates (glucose tablets, juice, regular soda).
Monitoring: More frequent blood sugar checking may be needed initially, especially if on insulin.
The risk of hypoglycemia is a good problem to have in some sense. It means your insulin resistance is improving enough that previous medication doses are now too much.
Duration of Treatment
Ongoing treatment is typically necessary. Insulin resistance typically worsens again if semaglutide is stopped and weight regains. Planning for long-term treatment aligns expectations with reality.
Potential for medication reduction: Some patients on multiple diabetes medications may eventually simplify their regimen while maintaining good control.
Monitoring over time: Regular lab checks ensure continued benefit and guide any adjustments.
Frequently Asked Questions
Does Ozempic directly improve insulin resistance or just help through weight loss?
Both. Semaglutide improves insulin resistance through direct metabolic effects and indirect effects from weight loss. The direct effects include enhanced insulin secretion (when blood sugar is elevated), suppressed glucagon release, reduced liver glucose production, and improved cellular response to insulin. These effects begin working within days, often improving blood sugar before significant weight loss occurs. The weight loss effects then amplify these benefits by reducing visceral fat, decreasing inflammation, and lowering the metabolic burden on the system. Research suggests the direct effects account for meaningful improvement independent of weight loss, though maximum benefit comes from both mechanisms working together.
How quickly will I see improvements in my insulin resistance on Ozempic?
Some improvements begin within the first few weeks. Many patients notice more stable blood sugar and reduced post-meal spikes within one to two weeks as the medication begins slowing gastric emptying and improving glucose-dependent insulin secretion. Fasting glucose often shows measurable improvement within the first month. However, HbA1c, which reflects average glucose over 2-3 months, takes longer to show the full effect of treatment, typically showing maximum improvement around months 3-6. Improvements in fasting insulin levels and HOMA-IR (a calculated measure of insulin resistance) parallel these glucose changes. Weight loss contributions to insulin sensitivity improvement compound over time as more weight is lost.
Can Ozempic reverse prediabetes?
For many patients, yes. Semaglutide can help patients with prediabetes return to normal glucose levels. By improving insulin sensitivity and producing significant weight loss, the medication addresses the underlying dysfunction driving prediabetes. Clinical evidence shows many prediabetic patients achieve normal fasting glucose (under 100 mg/dL) and normal HbA1c (under 5.7%) with treatment. This effectively means reversal of prediabetes, though continued treatment or significant lifestyle modification is typically needed to maintain these gains. For patients motivated by preventing progression to Type 2 diabetes, early intervention with semaglutide while still in the prediabetic stage offers the best chance of achieving and maintaining normal metabolic function.
How does Ozempic compare to metformin for insulin resistance?
Semaglutide generally produces greater improvement in insulin resistance than metformin. Clinical comparisons show semaglutide reduces HbA1c by approximately 1.0-1.6 percentage points versus 0.5-1.0 for metformin. Weight loss is dramatically different: 10-15% with semaglutide versus 2-5% with metformin. The cardiovascular benefits of semaglutide are more clearly established. However, metformin remains valuable because it costs dramatically less ($4-20/month versus $199-349), has decades of safety data, and can be combined with semaglutide. Many providers use both medications: metformin as affordable first-line treatment, with semaglutide added when additional improvement is needed or weight loss is prioritized.
Will I be able to reduce my other diabetes medications if I start Ozempic?
Many patients do reduce or eliminate other diabetes medications. As insulin resistance improves and blood sugar decreases, previously necessary medications may cause hypoglycemia (low blood sugar), requiring dose reductions. Patients on insulin frequently reduce doses, sometimes substantially, and some discontinue insulin entirely. Patients on sulfonylureas (like glipizide) often reduce or stop these medications. The extent of medication reduction depends on your starting point, how well you respond to semaglutide, and your baseline diabetes severity. These adjustments must be made with your healthcare provider’s guidance, not independently. The goal is achieving good glucose control with the simplest, safest medication regimen.
Is Ozempic better than lifestyle changes for insulin resistance?
Semaglutide produces substantially more improvement than lifestyle intervention alone for most patients. Clinical trials show average weight loss of 15% with semaglutide versus 5-7% with intensive lifestyle programs. The metabolic improvements parallel this difference. However, this doesn’t mean lifestyle changes don’t matter. The best outcomes come from combining medication with lifestyle modification. Semaglutide makes lifestyle changes easier by reducing appetite and food preoccupation, while the lifestyle changes maximize what the medication achieves. Additionally, lifestyle factors (diet quality, exercise, sleep, stress management) affect health through mechanisms beyond what any medication can address. The practical answer: semaglutide works much better than lifestyle changes alone, but combining both produces the best results.
What lab values should I track to monitor my insulin resistance?
Key labs include HbA1c (reflecting average blood sugar over 2-3 months), fasting glucose (baseline blood sugar after overnight fast), fasting insulin (how hard your pancreas is working), and lipid panel (particularly triglycerides, which correlate with insulin resistance). Your provider might also calculate HOMA-IR from fasting glucose and insulin to quantify insulin resistance directly. Liver enzymes (ALT, AST) are worth tracking since fatty liver disease is associated with insulin resistance and typically improves with treatment. Before starting treatment, establish baseline values. Recheck at 3 months, 6 months, and periodically thereafter. Beyond labs, track weight, waist circumference, blood pressure, and subjective factors like energy levels.
If I have insulin resistance but not diabetes, is Ozempic appropriate?
Yes, for patients who meet eligibility criteria. Insulin resistance without diabetes (often with prediabetes) is a valid reason to consider semaglutide, particularly if you also have excess weight. PCOS with insulin resistance similarly qualifies. From a metabolic health perspective, treating insulin resistance before it progresses to diabetes makes sense. You’re addressing the problem while intervention can be most effective. Standard eligibility requires BMI of 30 or higher, or BMI of 27 or higher with a weight-related condition. Insulin resistance and prediabetes count as qualifying conditions. The medication isn’t FDA-approved specifically for prediabetes, but off-label prescribing for this indication is common and medically reasonable.
What happens to my insulin resistance if I stop taking Ozempic?
For most patients, insulin resistance worsens again if semaglutide is stopped, particularly if weight regains. The medication manages insulin resistance rather than curing it. Research shows patients who stop semaglutide typically regain about two-thirds of lost weight within a year. As weight returns, so does the associated insulin resistance. Blood sugar rises, and metabolic markers worsen. This pattern reflects the chronic nature of obesity and insulin resistance. The biological factors driving these conditions don’t disappear after treatment. Planning for ongoing treatment rather than viewing semaglutide as temporary is more realistic. This has practical implications for cost sustainability and long-term medication planning.
Can Ozempic help if my insulin resistance is genetic?
Semaglutide can help even with genetically driven insulin resistance, though the degree of improvement may vary. Genetic factors influence insulin resistance through various mechanisms, some of which semaglutide addresses directly or indirectly. Weight loss improves insulin sensitivity regardless of the underlying cause. The direct metabolic effects of GLP-1 agonism improve glucose handling through mechanisms that work independently of the original cause of resistance. However, some genetic forms of insulin resistance may be less responsive than acquired, lifestyle-related resistance. Additionally, genetic factors affecting medication metabolism could influence individual response. The only way to know how you’ll respond is to try treatment and monitor your metabolic markers over time.
Does insurance cover Ozempic for insulin resistance without a diabetes diagnosis?
Coverage varies significantly by plan. Without a Type 2 diabetes diagnosis, getting Ozempic covered is more difficult since its FDA approval is for diabetes. Wegovy (the same medication in higher doses approved for weight management) may be covered by some plans for weight loss indications, though many plans exclude weight loss medications. Some patients find that a prediabetes diagnosis helps with coverage since this represents a diabetes-related indication. Prior authorization is typically required regardless. Many patients with insulin resistance but without formal diabetes diagnosis end up paying cash. Compounded semaglutide at $199/month through TrimRx provides an accessible option when insurance coverage isn’t available.
Taking the Next Step
Insulin resistance sits at the core of many metabolic problems, and addressing it can produce improvements that ripple across multiple aspects of health. Semaglutide offers a powerful tool for this purpose, working through both direct metabolic effects and weight loss to improve how your body handles glucose and responds to insulin.
The evidence supports meaningful improvements: better blood sugar control, reduced insulin levels, improved cholesterol and blood pressure, and reduced risk of progression from prediabetes to diabetes. For many patients, these benefits translate to reduced medication needs, improved energy, and better long-term health outlook.
Success requires consistent treatment, appropriate monitoring, and realistic expectations about timeline and outcomes. The medication makes improvement possible; working with your healthcare provider to optimize your individual response helps you achieve the best results.
Ready to explore whether semaglutide can help with your insulin resistance? TrimRx offers consultations with licensed providers who can evaluate your metabolic profile and prescribe compounded semaglutide at $199/month for qualifying patients.

Transforming Lives, One Step at a Time
Keep reading
Ozempic for Emotional Eating: Does It Help?
Emotional eating is one of the most common and least discussed barriers to sustainable weight loss. For people who eat in response to stress,…
Hashimoto’s and Ozempic: Safety Considerations
Hashimoto’s thyroiditis is the most common autoimmune condition in the United States, affecting an estimated 14 million people, the majority of them women. It’s…
Ozempic and Anxiety: Side Effects and Interactions (2026)
Anxiety is one of the more nuanced topics that comes up in conversations about GLP-1 medications. Some people report that starting Ozempic worsened their…