Semaglutide Compound Pharmacy: How Compounding Works and Quality Standards

If you’re considering compounded semaglutide, you likely have questions about where it comes from, how it’s made, and whether it’s safe. The compounding pharmacy industry operates under specific regulations that most consumers know little about, and understanding these standards helps you make informed decisions about your treatment.
Compounding pharmacies create customized medications by combining pharmaceutical ingredients according to prescriptions. For semaglutide, this means producing the same active molecule found in Ozempic and Wegovy, but outside the brand-name manufacturer’s supply chain. Legitimate compounding is a well-established practice with clear regulatory oversight, but quality varies between facilities, and understanding the differences matters for your safety.
The landscape includes two main types of compounding facilities: 503A pharmacies that fill individual prescriptions, and 503B outsourcing facilities that produce larger quantities under more stringent manufacturing standards. Both operate legally under FDA guidance, but their oversight, testing requirements, and quality systems differ significantly.
This guide covers:
- What compounding pharmacies are and why they exist
- How semaglutide compounding works
- The difference between 503A and 503B facilities
- Quality standards and testing requirements
- FDA oversight of compounding
- How to evaluate a compounding pharmacy’s quality
- Red flags that indicate problematic providers
- What legitimate compounded semaglutide looks like
- Questions to ask before starting treatment
Key Takeaways
- Compounding is legal and regulated under FDA guidance, with specific rules for how pharmacies can produce semaglutide
- 503A pharmacies fill individual prescriptions and are primarily state-regulated
- 503B outsourcing facilities produce larger quantities under stricter FDA oversight and cGMP standards
- Semaglutide can be compounded because the brand-name products have been on the FDA drug shortage list
- Quality varies significantly between compounding facilities; not all are equivalent
- Legitimate compounders use pharmaceutical-grade semaglutide from qualified suppliers and test their products
- Certificates of Analysis document purity, potency, and sterility testing for compounded products
- Red flags include unusually low prices, no prescription requirement, overseas sourcing, and lack of transparency
- Your prescribing provider should work with reputable compounding pharmacies with verified quality standards
- TrimRx partners with 503B facilities that meet rigorous quality and testing requirements
What Are Compounding Pharmacies?
Compounding pharmacies have existed for centuries, though their role has evolved as pharmaceutical manufacturing has industrialized.
The Basics of Compounding
Definition: Compounding is the process of creating customized medications by combining, mixing, or altering pharmaceutical ingredients to meet specific patient needs.
Historical context: Before mass pharmaceutical manufacturing, all medications were compounded. Pharmacists mixed ingredients to create each prescription. As drug manufacturing scaled up, most medications became commercially available, but compounding remained important for specific situations.
Modern role: Today, compounding serves patients whose needs aren’t met by commercially available products:
- Patients needing different dosages than available
- Patients allergic to inactive ingredients in commercial products
- Patients requiring different forms (liquid instead of pill, for example)
- Situations where commercial products are unavailable or in shortage
Why Semaglutide Is Compounded
Semaglutide compounding exists primarily because of:
Drug shortage status:
- Ozempic and Wegovy have been on the FDA’s drug shortage list
- When brand-name medications are in shortage, compounding is permitted
- This allows patients to access needed medications despite supply issues
Cost accessibility:
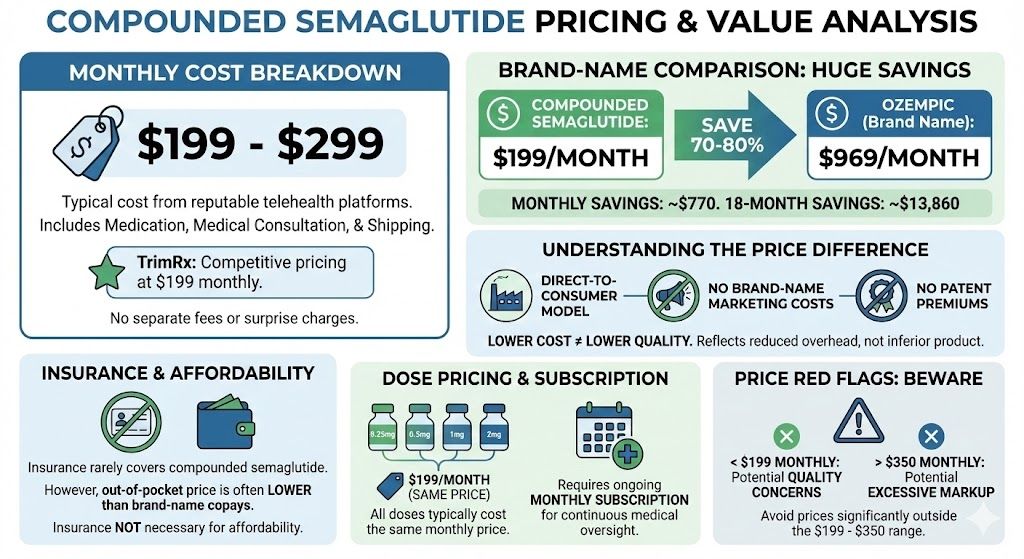
- Brand-name semaglutide costs $900-1,350/month at list price
- Even with manufacturer programs ($349/month), cost remains significant
- Compounded semaglutide (typically $199-299/month) provides more affordable access
Insurance gaps:
- Many insurance plans don’t cover weight loss medications
- Compounded versions provide access for uninsured or underinsured patients
Legal Framework
Compounding pharmacies operate under specific legal authority:
Federal Food, Drug, and Cosmetic Act (FDCA):
- Section 503A covers traditional compounding pharmacies
- Section 503B covers outsourcing facilities
- Both sections provide exemptions from certain FDA requirements for legitimate compounding
State pharmacy boards:
- License and regulate pharmacies within their states
- Enforce pharmacy practice standards
- Conduct inspections and handle complaints
FDA oversight:
- Sets standards for compounding practice
- Inspects facilities (especially 503B)
- Takes action against unsafe compounders
- Maintains drug shortage list that enables compounding
The Two Types of Compounding Facilities
Understanding the difference between 503A and 503B facilities is crucial for evaluating compounded medication quality.
503A Compounding Pharmacies
What they are:
- Traditional compounding pharmacies
- Fill prescriptions for individual patients
- Named after Section 503A of the FDCA
Regulatory framework:
- Primarily state-regulated through pharmacy boards
- Must have valid prescription before compounding
- Limited quantity restrictions
- Cannot compound drugs that are “essentially copies” of commercial products (with exceptions)
Oversight:
- State pharmacy board inspections
- FDA can inspect but doesn’t routinely
- Quality systems vary by facility
- Testing requirements vary by state
Characteristics:
- Often smaller operations
- May compound many different medications
- Quality depends heavily on individual pharmacy practices
- May or may not conduct extensive testing
For semaglutide:
- Can compound for individual patients with prescriptions
- Must comply with state regulations
- Quality and testing practices vary significantly
503B Outsourcing Facilities
What they are:
- Registered outsourcing facilities that compound larger quantities
- Named after Section 503B of the FDCA (created in 2013)
- Can supply healthcare facilities and providers
- Subject to stricter federal oversight
Regulatory framework:
- Must register with FDA
- Subject to current Good Manufacturing Practice (cGMP) standards
- FDA inspections similar to pharmaceutical manufacturers
- More stringent quality requirements
Oversight:
- Regular FDA inspections
- Must report adverse events to FDA
- Must meet cGMP standards
- More rigorous documentation requirements
Characteristics:
- Often larger, more sophisticated operations
- Dedicated quality systems
- Required testing and documentation
- More similar to pharmaceutical manufacturers
For semaglutide:
- Can produce larger quantities
- Subject to stricter quality standards
- More consistent quality controls
- Generally considered more reliable for sterile preparations
Comparing 503A and 503B
| Factor | 503A Pharmacy | 503B Outsourcing Facility |
| Primary regulation | State pharmacy boards | FDA |
| Manufacturing standards | State-dependent | cGMP required |
| FDA registration | Not required | Required |
| FDA inspections | Infrequent | Regular |
| Quantity limits | Generally smaller | Can produce larger quantities |
| Prescription requirement | Required before compounding | Not required for each batch |
| Testing requirements | State-dependent | More stringent |
| Adverse event reporting | State-dependent | Required |
Which Is Better?
503B advantages:
- More consistent federal oversight
- Required cGMP compliance
- More rigorous testing standards
- Generally more sophisticated quality systems
503A considerations:
- Some 503A pharmacies maintain excellent quality
- Can provide personalized service
- May offer customization options
- Quality varies more widely
For sterile injectables like semaglutide: Many experts consider 503B facilities more appropriate for sterile preparations because of their stricter quality requirements. Sterile compounding carries inherent risks, and more rigorous oversight provides additional safety assurance.
How Semaglutide Compounding Works
Understanding the compounding process helps you evaluate quality claims.
The Active Ingredient
Semaglutide API (Active Pharmaceutical Ingredient):
- The same molecule found in Ozempic and Wegovy
- Must be sourced from qualified suppliers
- Should meet pharmaceutical-grade purity standards
- Comes as a powder that requires reconstitution
Sourcing:
- Legitimate compounders obtain semaglutide from FDA-registered or inspected facilities
- Suppliers should provide Certificates of Analysis
- Quality of API directly affects quality of final product
- Reputable sources include manufacturers with FDA registration or equivalent international certification
The Compounding Process
For injectable semaglutide:
- Ingredient verification:
- API identity confirmed through testing
- Purity verified against specifications
- Documentation reviewed
- Calculation:
- Precise amounts calculated for target concentration
- Excipients (inactive ingredients) measured
- Final volume determined
- Sterile compounding:
- Performed in cleanroom environment
- Aseptic technique throughout
- Sterile equipment and containers
- Environmental monitoring
- Quality testing:
- Potency testing (correct amount of semaglutide)
- Sterility testing (no bacterial contamination)
- Endotoxin testing (no bacterial toxins)
- pH and other specifications
- Packaging and labeling:
- Appropriate vials or syringes
- Proper labeling with concentration, expiration, storage
- Secure packaging for shipping
Quality Testing
Critical tests for compounded semaglutide:
Potency testing:
- Confirms the stated amount of semaglutide is present
- Typically uses HPLC (High-Performance Liquid Chromatography)
- Should be within acceptable range (typically 90-110% of label claim)
Sterility testing:
- Confirms no microbial contamination
- Uses USP-compliant testing methods
- Critical for injectable products
Endotoxin testing:
- Tests for bacterial toxins
- Important even if sterility test passes
- Uses LAL (Limulus Amebocyte Lysate) testing
Visual inspection:
- Checks for particulates
- Confirms clarity and color
- Identifies any visible contamination
pH testing:
- Ensures proper acidity/alkalinity
- Affects stability and tolerability
Beyond-Use Dating
What it is:
- The date after which compounded product shouldn’t be used
- Different from expiration dating on commercial products
- Based on stability data and compounding standards
For compounded semaglutide:
- Typically 30-90 days depending on formulation and storage
- Based on stability studies
- Proper storage (refrigeration) is essential
- Shorter than brand-name products (which have extensive stability data)
Quality Standards in Compounding
Multiple standards govern compounding quality.
United States Pharmacopeia (USP) Standards
USP sets quality standards for pharmaceutical compounding:
USP Chapter <797>:
- Covers sterile compounding
- Sets standards for facilities, personnel, procedures
- Defines contamination risk levels
- Specifies environmental monitoring
- Requires competency testing for personnel
USP Chapter <800>:
- Covers hazardous drug handling
- May apply to some compounding situations
USP Chapter <795>:
- Covers non-sterile compounding
- Less relevant for injectable semaglutide
What USP compliance means:
- Facility meets environmental standards
- Personnel are trained and competency-tested
- Procedures follow established protocols
- Quality systems are in place
Current Good Manufacturing Practice (cGMP)
What cGMP means:
- FDA’s quality standards for pharmaceutical manufacturing
- Required for 503B outsourcing facilities
- Covers all aspects of production and quality
Key cGMP requirements:
- Qualified personnel with defined responsibilities
- Adequate facilities and equipment
- Written procedures for all operations
- Quality control testing
- Documentation of everything
- Complaint handling and adverse event reporting
- Investigations of quality problems
Why cGMP matters:
- More comprehensive than basic compounding standards
- Similar to requirements for pharmaceutical manufacturers
- Provides greater quality assurance
- Required FDA inspections verify compliance
State Pharmacy Board Standards
Role of state boards:
- License pharmacies in their states
- Set state-specific compounding requirements
- Conduct inspections
- Handle complaints and enforcement
Variation between states:
- Some states have stricter requirements than others
- Testing requirements vary
- Inspection frequency varies
- This creates quality variation among 503A pharmacies
FDA Oversight of Compounding
Understanding FDA’s role clarifies how compounding is regulated.
FDA’s Authority
For 503A pharmacies:
- FDA can inspect but doesn’t routinely
- Takes action when problems are identified
- Provides guidance on compounding standards
- Can take enforcement action against unsafe practices
For 503B facilities:
- FDA registration required
- Regular FDA inspections
- Must report adverse events
- Subject to enforcement for cGMP violations
The Drug Shortage Connection
Why shortage status matters:
- Normally, compounders can’t make “essentially copies” of approved drugs
- Drug shortage status provides an exception
- FDA maintains the drug shortage list
- Semaglutide’s presence on this list enables legal compounding
Current status:
- Wegovy and Ozempic have been on the shortage list
- This permits compounding of semaglutide
- Status can change as supply improves
- Compounders must monitor shortage status
FDA Enforcement Actions
Types of action:
- Warning letters for violations
- Import alerts for foreign suppliers
- Injunctions to stop operations
- Criminal prosecution in severe cases
- Product recalls
Recent concerns:
- FDA has warned about some semaglutide products
- Concerns include products containing salts (semaglutide sodium) not approved in branded products
- Some products have failed testing
- FDA has issued safety communications
What this means for consumers:
- Not all compounded semaglutide is equivalent
- Working with reputable providers matters
- FDA oversight provides some protection
- Vigilance is still important
How to Evaluate a Compounding Pharmacy
Not all compounding pharmacies are equal. Here’s how to assess quality.
Key Questions to Ask
About the facility:
- Are you a 503A pharmacy or 503B outsourcing facility?
- Are you registered with the FDA (for 503B)?
- What accreditations do you hold (PCAB, ACHC)?
- When was your last regulatory inspection?
About semaglutide specifically:
- Where do you source your semaglutide API?
- Is it pharmaceutical grade from an FDA-registered supplier?
- What testing do you perform on finished products?
- Can you provide Certificates of Analysis?
About quality systems:
- Do you follow USP <797> standards?
- Do you have cGMP compliance (for 503B)?
- What is your beyond-use dating and how is it determined?
- How do you handle quality complaints?
Accreditations to Look For
PCAB (Pharmacy Compounding Accreditation Board):
- Voluntary accreditation program
- Comprehensive quality standards
- Third-party verification of practices
- Demonstrates commitment to quality
ACHC (Accreditation Commission for Health Care):
- Accredits various healthcare organizations
- Includes pharmacy accreditation
- Third-party quality verification
State licenses:
- Required for legal operation
- Can verify with state pharmacy board
- Check for any disciplinary actions
Red Flags to Watch For
Pricing concerns:
- Prices significantly below market (below $100/month suggests corners being cut)
- Prices that seem too good to be true usually are
Regulatory concerns:
- No prescription required
- Shipping from overseas
- Unable to provide facility information
- No verifiable pharmacy license
Quality concerns:
- No testing mentioned or available
- Can’t provide Certificates of Analysis
- Vague about sourcing
- No beyond-use dating
Transparency concerns:
- Unwilling to answer questions
- No physical address
- No pharmacist available
- Anonymous or unclear ownership
What Good Quality Looks Like
Reputable compounders typically:
- Provide clear information about their facility
- Source from verified suppliers
- Test finished products
- Provide Certificates of Analysis on request
- Have verifiable licenses and registrations
- Employ licensed pharmacists
- Follow USP standards (and cGMP for 503B)
- Have clear beyond-use dating
- Store and ship products properly
The Semaglutide Sodium Concern
Recent FDA communications have raised concerns about certain compounded products.
What Happened
FDA warning (2024):
- FDA issued statements about compounded semaglutide products
- Particular concern about “semaglutide sodium” and “semaglutide acetate”
- These are salt forms of semaglutide
- Different from the base form in Ozempic/Wegovy
Why Salt Forms Are Concerning
Base vs. salt forms:
- Ozempic and Wegovy contain semaglutide base
- Some compounders have used semaglutide salts
- Salt forms have different molecular weights
- Dosing equivalence is not straightforward
Safety concerns:
- Not the same as what was studied in clinical trials
- Absorption and efficacy may differ
- Unknown safety profile for the salt forms
- Not approved by FDA in any product
What This Means for Consumers
Questions to ask:
- Does your product contain semaglutide base or a salt form?
- Is it the same form used in Ozempic/Wegovy?
- What does your Certificate of Analysis show?
What to avoid:
- Products listing “semaglutide sodium” or “semaglutide acetate”
- Providers who can’t clarify which form they use
- Products without clear documentation
What’s appropriate:
- Semaglutide base (same as branded products)
- Clear documentation of the form used
- Testing confirming identity and purity
Working With a Telehealth Provider
Many patients access compounded semaglutide through telehealth platforms.
How the Model Works
Typical process:
- Patient completes online consultation
- Provider evaluates eligibility
- Prescription written if appropriate
- Prescription sent to partner pharmacy
- Pharmacy compounds and ships medication
- Ongoing follow-up with provider
Provider responsibilities:
- Evaluate patient appropriateness
- Write valid prescriptions
- Select reputable pharmacy partners
- Provide ongoing medical supervision
Pharmacy responsibilities:
- Fill prescriptions according to standards
- Ensure quality of compounded products
- Ship properly
- Maintain proper storage
Evaluating a Telehealth Provider’s Pharmacy Partners
Questions for telehealth providers:
- Which pharmacy compounds your semaglutide?
- Is it a 503A or 503B facility?
- What quality standards do they follow?
- What testing do they perform?
- Can you provide documentation of quality?
What good providers should offer:
- Transparency about pharmacy partners
- Willingness to discuss quality standards
- Ability to provide documentation
- Clear information about the product
TrimRx’s Approach
Our pharmacy partnerships:
- We work with 503B outsourcing facilities
- Partners follow cGMP standards
- Products undergo rigorous testing
- We can provide quality documentation
Why we chose 503B:
- More stringent federal oversight
- Required cGMP compliance
- Better suited for sterile preparations
- More consistent quality assurance
Proper Storage and Handling
Compounded semaglutide requires appropriate storage.
Storage Requirements
Temperature:
- Refrigerate at 36-46°F (2-8°C)
- Do not freeze
- Protect from light
- Keep in original packaging until use
Why storage matters:
- Peptides like semaglutide are sensitive to temperature
- Improper storage can reduce potency
- Can affect stability and beyond-use dating
- Proper storage ensures you receive full benefit
Shipping Considerations
Cold chain shipping:
- Should ship with cold packs
- Insulated packaging
- Expedited shipping to minimize transit time
- Temperature indicators may be included
What to check on arrival:
- Packaging intact
- Cold packs still cool (not necessarily frozen, but cool)
- Product within temperature range
- No obvious problems with vials
After Opening
Multi-dose vials:
- Note the date opened
- Follow beyond-use dating (typically 28-30 days after opening)
- Continue to refrigerate
- Inspect before each use
What to look for:
- Clear solution (not cloudy or discolored)
- No visible particles
- No unusual smell
- Proper seal/stopper condition
Frequently Asked Questions
What is a semaglutide compound pharmacy?
A semaglutide compound pharmacy is a licensed facility that creates customized semaglutide preparations for patients. These pharmacies obtain pharmaceutical-grade semaglutide (the active ingredient) from qualified suppliers and compound it into injectable form according to prescriptions. Compounding pharmacies operate under FDA oversight and state pharmacy board regulations. They exist because brand-name semaglutide (Ozempic, Wegovy) has been in shortage and is expensive, creating a need for alternative access. Quality varies between facilities, so choosing a reputable compounder with proper testing and quality standards is important.
Is compounded semaglutide legal?
Yes, compounded semaglutide is legal when produced by properly licensed pharmacies following applicable regulations. The FDA permits compounding of medications that are on the drug shortage list, which has included semaglutide products. Compounding pharmacies must hold valid state licenses and follow federal and state regulations. Section 503A of the FDCA covers traditional compounding pharmacies, while Section 503B covers registered outsourcing facilities with stricter FDA oversight. The key requirements are a valid prescription, proper licensing, and following compounding standards.
What’s the difference between 503A and 503B compounding pharmacies?
503A pharmacies are traditional compounding pharmacies primarily regulated by state pharmacy boards. They fill individual prescriptions and have variable quality standards depending on state requirements and individual practices. 503B outsourcing facilities are FDA-registered, subject to regular FDA inspections, and must follow current Good Manufacturing Practice (cGMP) standards similar to pharmaceutical manufacturers. For sterile injectables like semaglutide, 503B facilities generally provide more consistent quality assurance due to stricter federal oversight, required testing, and more sophisticated quality systems. Both can legally compound semaglutide, but 503B facilities typically have more rigorous quality controls.
How do I know if compounded semaglutide is safe?
Safety depends on the quality of the compounding facility and their practices. Look for pharmacies that source semaglutide from FDA-registered suppliers, test their finished products for potency and sterility, can provide Certificates of Analysis, follow USP <797> standards (and cGMP for 503B facilities), and have proper state licenses and accreditations. Ask your provider about their pharmacy partners’ quality standards. Avoid providers who can’t answer quality questions, offer prices significantly below market rates, don’t require prescriptions, or ship from overseas. Working with reputable telehealth providers who vet their pharmacy partners adds an additional safety layer.
Is compounded semaglutide the same as Ozempic?
Compounded semaglutide contains the same active molecule as Ozempic and Wegovy when properly made. The semaglutide base should be identical. However, there are differences: brand-name products have extensive clinical trial data, precise manufacturing controls, and FDA approval for specific indications. Compounded versions may use different inactive ingredients, have shorter beyond-use dating, and lack the same regulatory review. Legitimate compounding produces an effective product, but it’s not identical in every way to branded products. Additionally, some compounders have used salt forms (semaglutide sodium) that differ from the base form in branded products, which is concerning.
Why is compounded semaglutide cheaper than Ozempic?
Compounded semaglutide is less expensive for several reasons. Compounders don’t have the research and development costs that Novo Nordisk invested in developing and testing the medication. They don’t have marketing expenses or the overhead of a large pharmaceutical company. They purchase bulk active ingredient from API manufacturers rather than producing it themselves. They don’t need to conduct clinical trials. They operate under different regulatory frameworks than FDA-approved drug manufacturers. These factors allow compounders to offer semaglutide at $199-299/month compared to brand-name prices of $900-1,350/month, while still maintaining appropriate quality standards.
What should a Certificate of Analysis show for compounded semaglutide?
A Certificate of Analysis (CoA) for compounded semaglutide should document key quality tests. Potency testing should confirm the stated amount of semaglutide is present (typically within 90-110% of label claim). Sterility testing should confirm no microbial contamination. Endotoxin testing should confirm acceptable levels of bacterial toxins. The CoA should identify the product, lot number, and date. It should specify the test methods used and the results. It should indicate whether each test passed specifications. Reputable compounders will provide CoAs on request, and your provider should be able to obtain this documentation.
Can compounded semaglutide be shipped to my home?
Yes, compounded semaglutide can be shipped directly to patients. However, proper cold chain shipping is essential because semaglutide is a peptide that requires refrigeration. Reputable pharmacies ship with cold packs, insulated packaging, and expedited delivery to maintain temperature control. When your package arrives, verify the cold packs are still cool and the product appears properly maintained. If packaging arrives damaged or warm, contact your provider or pharmacy before using. Some states may have specific requirements for pharmacy shipping, but most permit direct-to-patient delivery of compounded medications with valid prescriptions.
What’s the concern about semaglutide sodium?
The FDA has raised concerns about compounded products containing semaglutide sodium or semaglutide acetate rather than semaglutide base. Ozempic and Wegovy contain semaglutide base, the form studied in clinical trials. Salt forms have different molecular weights, potentially different absorption characteristics, and unknown safety profiles compared to the base form. They’re not equivalent milligram-for-milligram. The FDA has not approved any semaglutide salt forms. When choosing compounded semaglutide, verify the product contains semaglutide base, the same form used in branded products, and ask for documentation confirming this.
How do I verify a compounding pharmacy is legitimate?
To verify a compounding pharmacy, check several sources. Confirm their state pharmacy license with the relevant state board of pharmacy. For 503B facilities, verify FDA registration on the FDA’s registered outsourcing facilities list. Check for accreditations like PCAB (Pharmacy Compounding Accreditation Board). Look for any disciplinary actions or warning letters. Ask for their physical address and contact information. Legitimate pharmacies will readily provide this information. Be wary of pharmacies that can’t be verified, have no clear physical location, or are evasive about their credentials and practices.
Why does my telehealth provider use a specific compounding pharmacy?
Telehealth providers typically establish relationships with specific compounding pharmacies after vetting their quality standards, testing practices, and regulatory compliance. Good providers choose pharmacy partners based on quality credentials rather than solely on price. They may require pharmacies to meet certain standards (like 503B registration, specific testing protocols, or accreditations) before partnering. This vetting process provides patients additional assurance beyond what they could evaluate independently. Ask your provider what criteria they use to select pharmacy partners and what quality standards those partners meet.
The Bottom Line
Compounding pharmacies play a legitimate and regulated role in providing access to semaglutide for patients who cannot access or afford brand-name products. Understanding how this industry works helps you make informed decisions about your care.
Key points to remember:
Quality varies: Not all compounding pharmacies are equivalent. 503B outsourcing facilities operate under stricter federal oversight and cGMP requirements than traditional 503A pharmacies.
Due diligence matters: Ask questions about sourcing, testing, and quality standards. Reputable providers will answer these questions readily.
Red flags exist: Unusually low prices, no prescription requirements, overseas shipping, and lack of transparency all suggest potential quality concerns.
Your provider matters: Working with a telehealth provider who has vetted their pharmacy partners provides an additional layer of quality assurance.
At TrimRx, we partner with 503B outsourcing facilities that meet rigorous quality standards, including cGMP compliance and comprehensive product testing. Our compounded semaglutide is sourced from qualified suppliers and tested for potency, sterility, and purity.
Ready to explore semaglutide treatment? TrimRx offers consultations with licensed providers who can evaluate your eligibility and prescribe compounded semaglutide at $199/month from our quality-verified pharmacy partners.

Transforming Lives, One Step at a Time
Keep reading
Ozempic for Emotional Eating: Does It Help?
Emotional eating is one of the most common and least discussed barriers to sustainable weight loss. For people who eat in response to stress,…
Hashimoto’s and Ozempic: Safety Considerations
Hashimoto’s thyroiditis is the most common autoimmune condition in the United States, affecting an estimated 14 million people, the majority of them women. It’s…
Ozempic and Anxiety: Side Effects and Interactions (2026)
Anxiety is one of the more nuanced topics that comes up in conversations about GLP-1 medications. Some people report that starting Ozempic worsened their…



