Tirzepatide Contraindications: Who Should Avoid It?

Tirzepatide, known by its brand names Mounjaro (for type 2 diabetes) and Zepbound (for weight loss), is a powerful medication with proven results. However, it’s not safe for everyone. Here’s a quick breakdown of who should avoid it:
Key Contraindications:
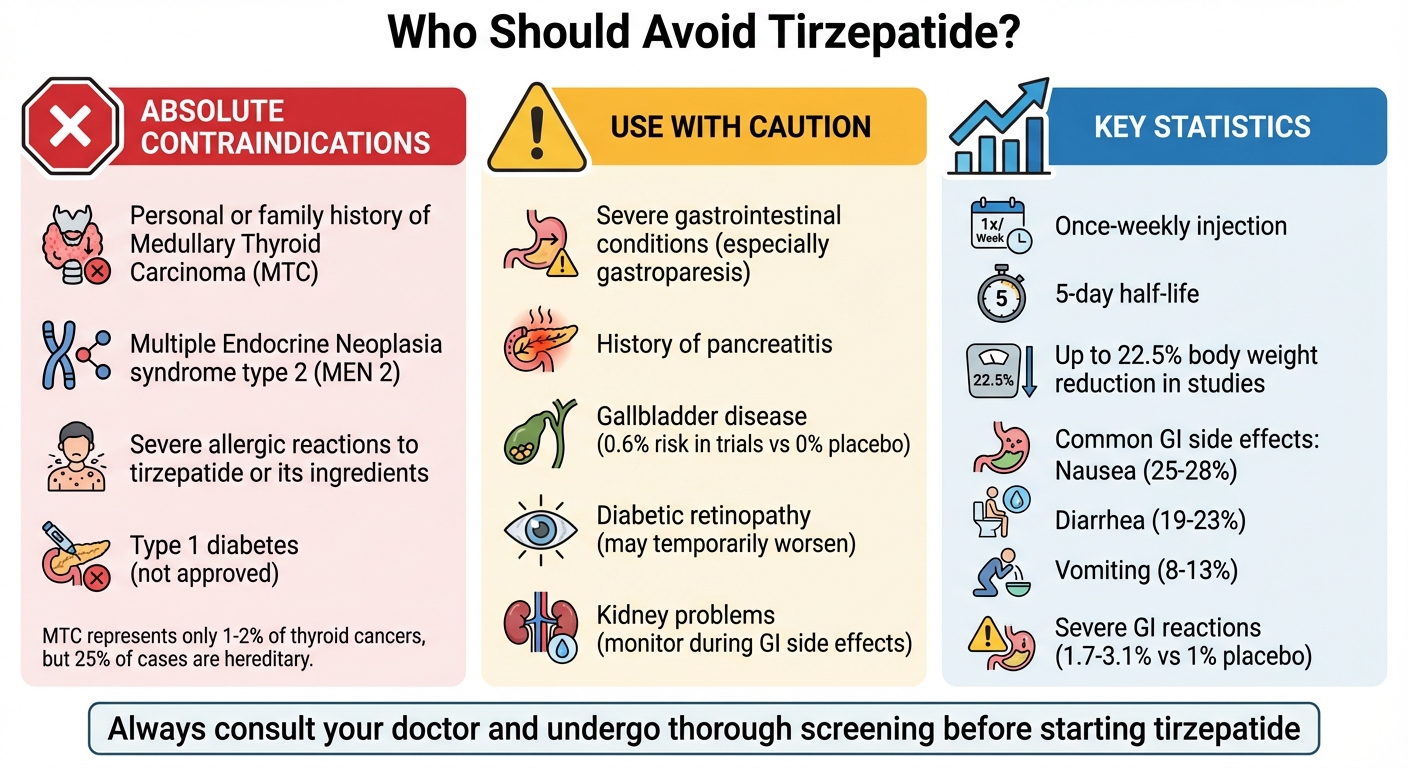
- Personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) due to potential thyroid tumor risks.
- Severe allergic reactions to tirzepatide or its ingredients.
- Severe gastrointestinal conditions, such as gastroparesis.
- Type 1 diabetes, as it’s not approved for this condition.
Use with Caution:
- Gallbladder disease, as rapid weight loss may increase gallstone risk.
- Diabetic retinopathy, which may worsen during rapid blood sugar improvements.
- Kidney issues, especially if dehydration occurs from side effects like nausea or vomiting.
Quick Facts:
- Tirzepatide is a once-weekly injection with a half-life of five days.
- It helps manage blood sugar, reduces A1C levels, and supports weight loss (up to 22.5% body weight reduction in studies).
- Always consult your doctor and undergo thorough screening to ensure it’s safe for you.
If tirzepatide isn’t suitable, alternatives like semaglutide or non-prescription weight loss options are available. Always prioritize safety and regular medical monitoring when considering this medication.
Tirzepatide Contraindications and Caution Conditions Guide
Ozempic & Tirzepatide Side Effects Explained (Doctor Reacts to the GLP-1 Data)
Absolute Contraindications: Who Cannot Use Tirzepatide
Tirzepatide is not suitable for everyone. Below are the key conditions that make its use unsafe.
Personal or Family History of Medullary Thyroid Carcinoma (MTC)
If you or a first-degree relative has had medullary thyroid carcinoma, tirzepatide is off the table. This restriction stems from findings in a 2-year study where the drug caused thyroid C-cell tumors in rats, regardless of the dose. While it’s unclear if this risk applies to humans, the FDA has issued a Boxed Warning to highlight the potential danger.
"Tirzepatide causes thyroid C-cell tumors in rats. It is unknown whether MOUNJARO causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined." – FDA Boxed Warning
MTC is rare, making up just 1% to 2% of all thyroid cancers. However, about 25% of cases are hereditary. Before starting tirzepatide, your doctor should ask about any family history of thyroid cancer. If you notice symptoms like a neck lump, persistent hoarseness, difficulty swallowing, or shortness of breath while on the medication, contact your doctor immediately.
Multiple Endocrine Neoplasia Syndrome Type 2 (MEN 2)
MEN 2 is a rare genetic condition that raises the risk of medullary thyroid carcinoma. It’s typically linked to mutations in the RET proto-oncogene and follows an autosomal dominant inheritance pattern. For individuals with MEN 2, tirzepatide poses an unacceptable risk because it has been shown to promote thyroid C-cell growth in animal studies.
"MOUNJARO is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)." – Eli Lilly and Company
If you or your family has a history of MEN 2, genetic testing can confirm the diagnosis. Your doctor should screen for MEN 2 before prescribing tirzepatide. Elevated serum calcitonin levels – typically above 50 ng/L – may suggest MTC, though routine calcitonin monitoring for all tirzepatide users is not currently recommended.
Severe Allergic Reactions to Tirzepatide or Its Ingredients
If you’ve had a severe allergic reaction to tirzepatide or its ingredients, the medication is not an option for you. Severe reactions, such as anaphylaxis or angioedema, are medical emergencies that require immediate attention.
Tirzepatide contains inactive ingredients like sodium chloride, sodium phosphate dibasic heptahydrate, and water for injection. Review this list with your doctor to identify any potential allergens. If you’ve experienced severe reactions to other GLP-1 receptor agonists, proceed with caution, as cross-reactivity is possible.
Signs of a serious allergic reaction include swelling of the face, lips, or tongue, difficulty breathing, or other severe symptoms. If these occur, stop the medication immediately and seek emergency care. Do not restart tirzepatide after such a reaction. Additionally, patients who develop anti-tirzepatide antibodies during treatment may face a higher likelihood of allergic or injection site reactions.
Conditions Requiring Caution with Tirzepatide
Tirzepatide, while effective for many, requires extra care in certain situations to ensure safety. Some conditions may call for close monitoring, dose adjustments, or a detailed discussion with your doctor about whether this medication is the right choice for you.
Severe Gastrointestinal Conditions
Tirzepatide slows down how quickly food leaves your stomach, which can be problematic for individuals with severe gastroparesis – a condition where the stomach already struggles to empty properly. The FDA specifically advises against using Zepbound in such cases:
"ZEPBOUND is not recommended in patients with severe gastroparesis." – FDA Prescribing Information
Gastrointestinal side effects, particularly during the dose escalation phase, are common. In clinical trials, severe reactions were reported in 1.7% of patients using 5 mg, 2.5% with 10 mg, and 3.1% with 15 mg, compared to only 1% for placebo. This led to treatment discontinuation in 1.9% to 4.3% of users depending on the dose.
If you have a history of pancreatitis, proceed with caution. Tirzepatide has been linked to acute pancreatitis, though it hasn’t been thoroughly studied in this group. If you experience persistent, severe abdominal pain, seek medical attention immediately, as this may indicate a serious complication.
To reduce gastrointestinal discomfort, follow the recommended dose escalation schedule, starting at 2.5 mg weekly for four weeks. Eating smaller, more frequent meals, avoiding high-fat or fried foods, and drinking at least 64 ounces of water daily can help. If you’re preparing for surgery, let your healthcare provider know you’re on tirzepatide – patients at higher risk may need to follow a liquid diet 24 hours before anesthesia to prevent complications like pulmonary aspiration.
| Adverse Reaction | Placebo (%) | Tirzepatide 5 mg (%) | Tirzepatide 10 mg (%) | Tirzepatide 15 mg (%) |
|---|---|---|---|---|
| Nausea | 8 | 25 | 29 | 28 |
| Diarrhea | 8 | 19 | 21 | 23 |
| Vomiting | 2 | 8 | 11 | 13 |
| Constipation | 5 | 17 | 14 | 11 |
Next, it’s important to consider conditions like type 1 diabetes, which also pose specific challenges.
Type 1 Diabetes and Other Non-Approved Uses
Tirzepatide is approved by the FDA for managing type 2 diabetes and aiding weight loss. However, it is not approved for type 1 diabetes, and using it for unapproved purposes can be risky. Its mechanism focuses on improving insulin resistance and regulating glucose, which doesn’t align with the needs of individuals whose pancreas produces little to no insulin.
For those with type 1 diabetes, tirzepatide cannot replace essential insulin therapy. Using it inappropriately could result in dangerous complications like diabetic ketoacidosis. Stick to medications designed for your condition, and consult your endocrinologist about any weight management concerns.
Other conditions, such as gallbladder issues, diabetic retinopathy, and kidney problems, also require careful consideration.
Gallbladder Disease, Diabetic Retinopathy, and Kidney Problems
Gallbladder disease is another concern with tirzepatide. The rapid weight loss it often causes can increase the risk of gallstones (cholelithiasis) and gallbladder inflammation (cholecystitis). In placebo-controlled trials, 0.6% of patients on tirzepatide experienced acute gallbladder disease, compared to none in the placebo group. Watch for signs like severe upper stomach pain radiating to your back, fever, yellowing of the skin or eyes, or pale stools. If these symptoms appear, contact your doctor immediately for evaluation and treatment.
Diabetic retinopathy may temporarily worsen with rapid blood sugar improvements during tirzepatide treatment. The FDA highlights this risk:
"Rapid improvement in glucose control has been associated with temporary worsening of diabetic retinopathy." – FDA
Report any changes in vision to your healthcare provider promptly for monitoring and intervention.
Kidney problems also warrant attention. While no dosage adjustment is needed based on kidney function alone, gastrointestinal side effects like nausea or vomiting can lead to dehydration, increasing the risk of acute kidney injury.
"Monitor renal function when initiating or escalating doses of tirzepatide in patients with renal impairment reporting severe GI adverse reactions." – Drugs.com Monograph
Stay hydrated and alert for signs of kidney issues, such as reduced urine output, blood in your urine, or swelling in your face and ankles. These precautions are essential for ensuring tirzepatide is used safely and effectively.
sbb-itb-e2779c3
How TrimRX Ensures Safe Use of Tirzepatide

TrimRX takes patient safety seriously by implementing strict evaluation and follow-up procedures to minimize risks when prescribing tirzepatide. Every patient undergoes a thorough health assessment to confirm they are a suitable candidate for the medication. For those who cannot use tirzepatide safely, TrimRX offers alternative options tailored to their needs.
Medical Consultations for Patient Eligibility
Before prescribing tirzepatide, TrimRX conducts detailed medical screenings to identify any conditions that could make the medication unsafe. Healthcare providers pay close attention to absolute contraindications like a personal or family history of Medullary Thyroid Carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). These conditions automatically disqualify patients due to significant safety concerns.
Eligibility criteria include a BMI of 30 kg/m² or higher, or 27 kg/m² or higher for those with weight-related conditions. During consultations, patients are asked about any history of gastrointestinal issues, kidney disease, or diabetic retinopathy, as these conditions may require extra caution and monitoring. Women using oral contraceptives are advised to consider alternative birth control methods while on tirzepatide. For patients who cannot safely take tirzepatide, TrimRX ensures they have access to other weight loss solutions.
Alternative Weight Loss Options
TrimRX provides a range of alternatives for patients who are not eligible for tirzepatide. One option is semaglutide, which is available in both injectable and oral forms. This medication supports weight loss by targeting GLP-1 receptors. Pricing for semaglutide starts at $199 for injectables and $179 for oral formulations, and includes free delivery, custom dosing, and ongoing healthcare support.
For those seeking non-prescription options, TrimRX offers supplements designed to complement weight loss efforts. The GLP-1 Daily Support supplement, priced at $119, includes Alpha Lipoic Acid, Berberine, Chromium, and a Multivitamin Blend tailored for GLP-1 users. Another option, the Weight Loss Boost supplement, costs $149 and is formulated to enhance fat burning and promote sustainable weight loss without requiring a prescription.
Continuous Medical Support and Monitoring
TrimRX ensures patients receive consistent support throughout their treatment journey. With unlimited check-ins available, patients can consult healthcare professionals whenever needed. This ongoing guidance helps manage side effects, adjust dosages, and address any concerns promptly. Providers offer advice on managing common issues like gastrointestinal discomfort, staying hydrated to protect kidney health, and recognizing symptoms that require immediate attention.
Conclusion
Understanding the risks associated with tirzepatide is crucial for ensuring safe and effective weight loss management. While tirzepatide has shown impressive results in aiding weight loss, it’s not suitable for everyone. Absolute contraindications include a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN 2), as well as severe allergic reactions to its components. Additionally, individuals with conditions like severe gastrointestinal disease, a history of pancreatitis, diabetic retinopathy, or kidney issues need thorough evaluation before starting treatment.
"Tirzepatide is contraindicated in patients with a personal or family history of MTC and in patients with multiple endocrine neoplasia syndrome type 2 (MEN 2)." – Drugs.com
A detailed medical screening is key to preventing complications. By identifying potential risks, healthcare providers can modify treatment plans or suggest safer alternatives, such as semaglutide or other tailored weight loss solutions.
At TrimRX, safety is a top priority. Through in-depth consultations, comprehensive health evaluations, and ongoing monitoring, every patient receives care tailored to their medical history, current health, and weight loss goals. With unlimited check-ins, any concerns are addressed promptly, and treatments are adjusted as needed to ensure the best outcomes.
Curious about your weight loss options? Take TrimRX’s free assessment quiz to see if you qualify and get a personalized plan crafted just for you.
FAQs
What should I do if I experience side effects while taking tirzepatide?
If you experience any side effects while using tirzepatide, reach out to your healthcare provider right away. They’ll evaluate your symptoms, decide if your dosage needs adjustment, or determine if you should discontinue the medication.
It’s important to closely follow your doctor’s instructions, which might include regular health check-ups or changes to your daily habits. Staying in touch with your provider helps ensure you manage any side effects effectively and prioritize your overall health.
Can tirzepatide be taken with other diabetes medications?
Tirzepatide can be taken along with other diabetes medications like metformin, but this should only happen under the supervision of a healthcare provider. Combining tirzepatide with other GLP-1 receptor agonists or products containing tirzepatide is not recommended, as it might cause side effects or reduce the medication’s effectiveness.
Make sure to consult your doctor to determine if tirzepatide fits into your treatment plan safely.
Can a family history of certain conditions make someone ineligible for tirzepatide?
Yes, a family history of certain conditions can influence whether someone is eligible to take tirzepatide. This medication is generally not recommended for individuals with either a personal or family history of medullary thyroid carcinoma (MTC) or a genetic predisposition to Multiple Endocrine Neoplasia type 2 (MEN 2). These conditions are associated with thyroid C-cell tumors, and animal studies have linked tirzepatide to such tumors. However, it’s not yet clear if the same risk applies to humans.
Before prescribing tirzepatide, healthcare providers usually assess patients for these risks and explain potential warning signs of thyroid-related issues. If there’s a family history of MTC, using tirzepatide may not be advised unless a detailed evaluation by a healthcare professional confirms it’s safe to move forward.
Related Blog Posts

Transforming Lives, One Step at a Time
Keep reading
How to Adjust Weight Loss Goals on GLP-1 Medications
Adjust weight goals on GLP-1 meds by tracking body composition and labs, optimizing protein, activity, sleep, and working with your provider on dosing.
Falsified Mounjaro pens prompt urgent safety advisory
MHRA warns of counterfeit Mounjaro pens (batch D873576); stop use and check batch numbers for infection risk.
Novo Nordisk shares drop as Alzheimer’s hopes for weight-loss drug fade
Novo Nordisk’s semaglutide failed to slow Alzheimer’s in large trials, prompting shares to fall and expert reactions.